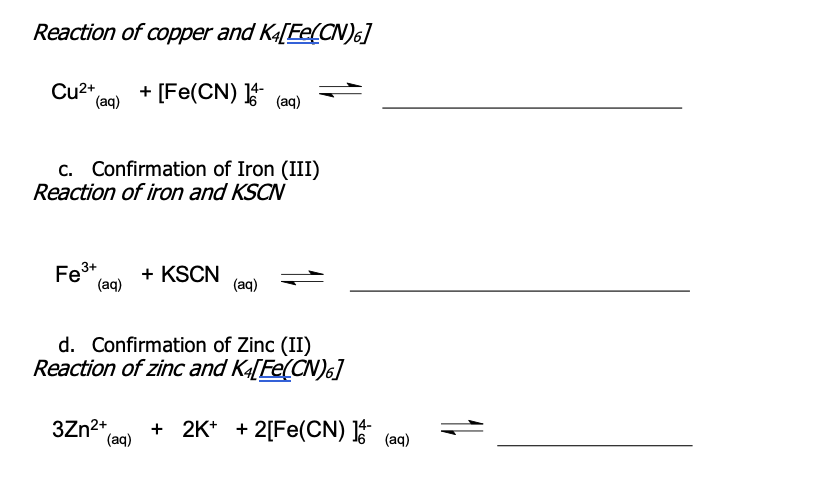
Question: Write the balanced net ionic equations for the following procedure: Reaction of copper and K4[Fe(CN)6] Cu2+(aq)+[Fe(CN)]64(aq)= c. Confirmation of Iron (III) Reaction of iron and
 Write the balanced net ionic equations for the following procedure:
Write the balanced net ionic equations for the following procedure:
Reaction of copper and K4[Fe(CN)6] Cu2+(aq)+[Fe(CN)]64(aq)= c. Confirmation of Iron (III) Reaction of iron and KSCN Fe(aq)3++KSCN(aq) d. Confirmation of Zinc (II) Reaction of zinc and K4[Fe(CN)6] 3Zn2+(aq)+2K++2[Fe(CN)]64(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


