Question: Write the different equations for the lines that separate the areas of corrosion, passivation and immunity using the Pourbaix Diagram. HOMEWORK: Write the different equations
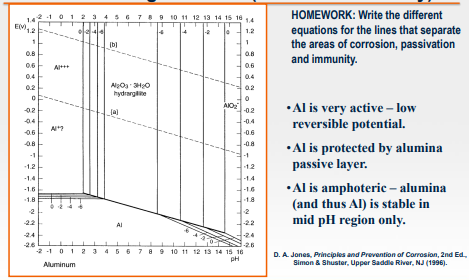
Write the different equations for the lines that separate the areas of corrosion, passivation and immunity using the Pourbaix Diagram.
HOMEWORK: Write the different equations for the lines that separate the areas of corrosion, passivation and immunity. - Al is very active - low reversible potential. - Al is protected by alumina passive layer. - Al is amphoteric - alumina (and thus Al ) is stable in mid pH region only. D. A. Jones, Principios avad Prevention of Corrosion, 2 nd Ed. Simon \& Bhuster, Upper Baddle Rher, NJ (1996)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


