Question: Write the empirical formula for at least four ionic compounds that could be formed from the following ions: 2+ 4+ Fe*, C,H,O, co , Pb
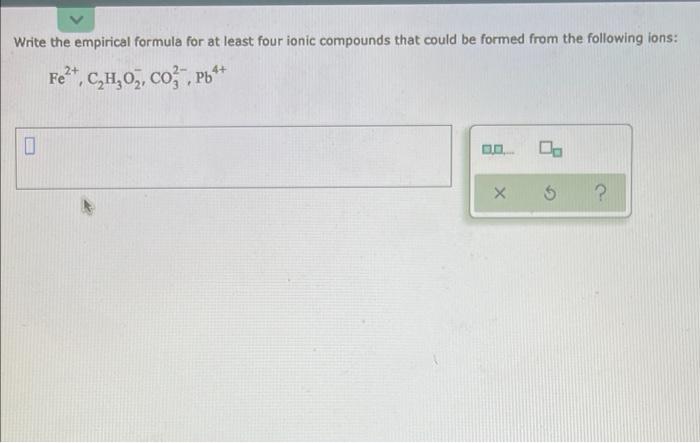
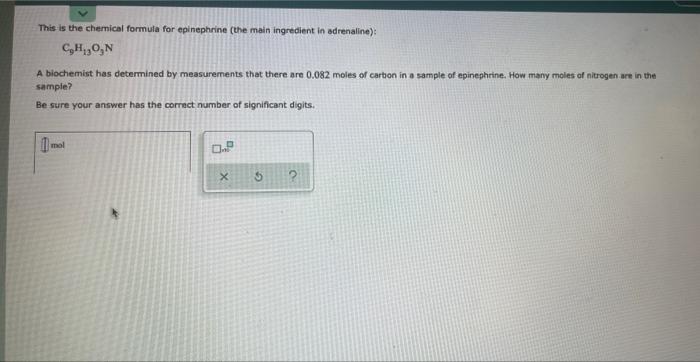
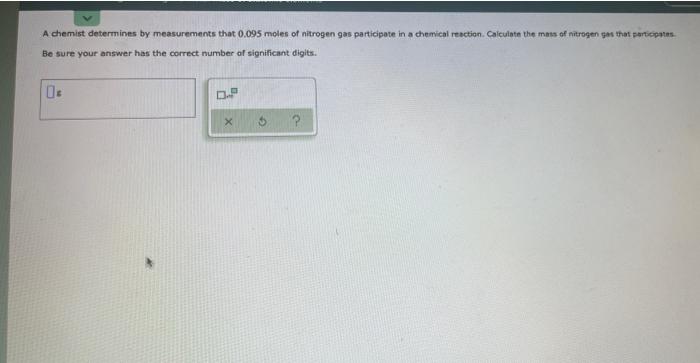
Write the empirical formula for at least four ionic compounds that could be formed from the following ions: 2+ 4+ Fe*, C,H,O, co , Pb + " 0 0. 5 ? This is the chemical formula for epinephrine (the main ingredient in adrenaline): C,H,O,N A biochemist has determined by measurements that there are 0,082 moles of cartion in a sample of epinephrine. How many moles of nitrogen are in the sample? Be sure your answer has the correct number of significant digits. 0.- 5 2 A chemist determines by measurements that 0.095 moles of nitrogen gas participate in a chemical reaction. Calculate the mass of nitrogen ges that participate Be sure your answer has the correct number of significant digits. 0: D.P 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


