Question: Write the expected ground-state electron configuration for the following. (a) the element with one unpaired Sp electron that forms a covalent compound with fluorine chemPad
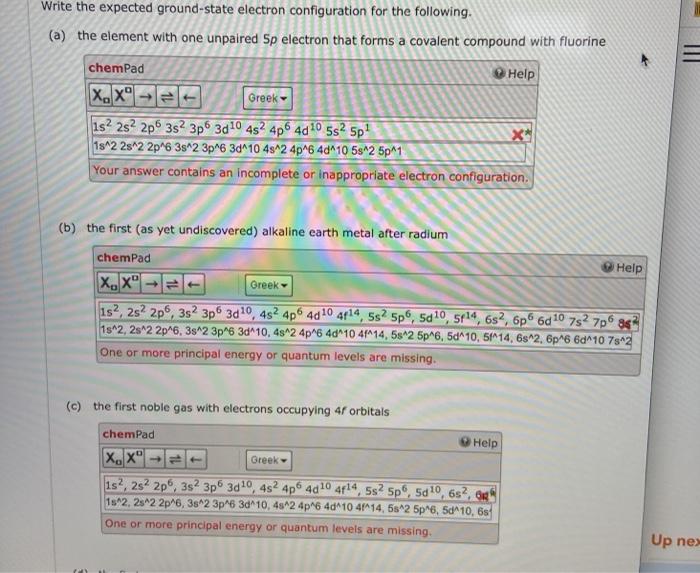
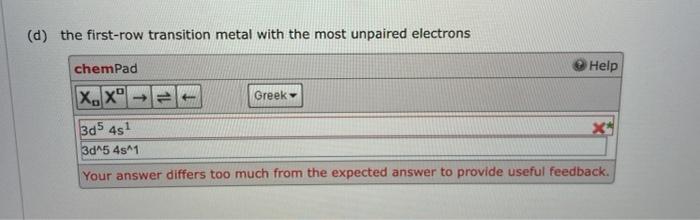
Write the expected ground-state electron configuration for the following. (a) the element with one unpaired Sp electron that forms a covalent compound with fluorine chemPad Help X. Xol Greek is? 2s 2p 3s 3p 30 10 482 4p6 40 10 5s2 Sp! 1s^2 2s 2 2p^6 3s^2 3p6 3d410 4s^24p^6 4d 10 5s^2 5p41 Your answer contains an incomplete or inappropriate electron configuration. (b) the first (as yet undiscovered) alkaline earth metal after radium chemPad Help XX1 Greek 1s2, 2s 2p6, 352 3p6 3d10,452 4p 4010 4414, 5s? 5p6, 5d10, 5r14, 632, 6p6d 10 782 7p8s? 1s2, 2s 2 2p 6, 3s^2 3p 6 3d"10, 4s^2 4p^6 4d410 4114, 582 5p6, 5d^10, 51M14, 6-2, 6p6 6d 10 7842 One or more principal energy or quantum levels are missing. (c) the first noble gas with electrons occupying 4 orbitals chemPad Help X.X Greek 1s?, 2s 2p 3s 3p 3d10,4s24p 4010 4r14, 5s2 Sp, 5d10, 6s, 6 1s 2. 2s 2 2p 6, 382 3p 6 3d 10,4s24p6 4d410 4114, 58^2 5p 6, 5d-10.6s One or more principal energy or quantum levels are missing. Up ne (d) the first-row transition metal with the most unpaired electrons chemPad Help (x,x Greek 13d5 4st 3d^5 481 Your answer differs too much from the expected answer to provide useful feedback
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


