Question: Write the formulas for the following ionic compounds: 6) potassium iodide 7) magnesium oxide 8) aluminum chloride 9) sodium nitrate 10) calcium carbonate 11) lithium

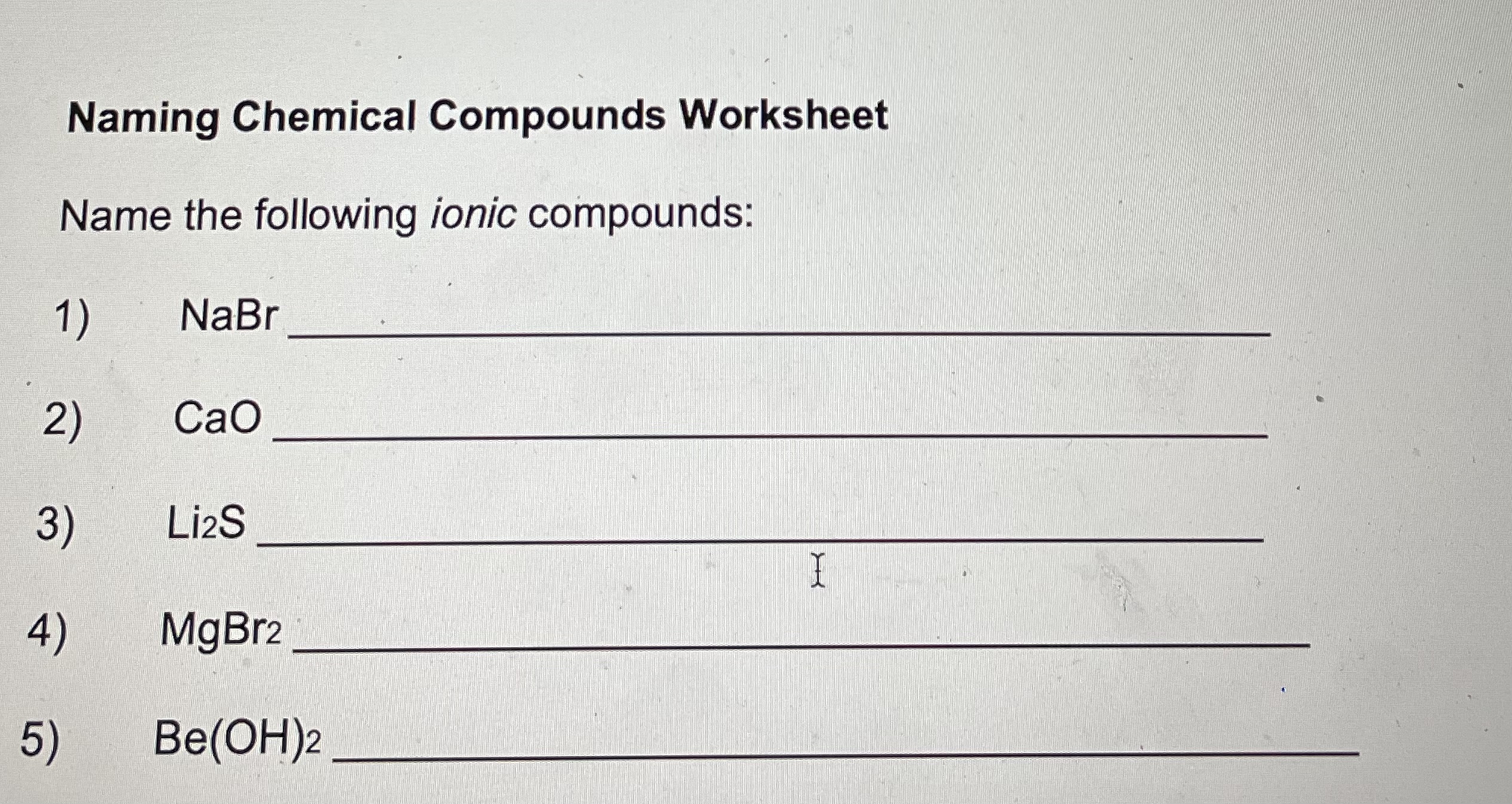
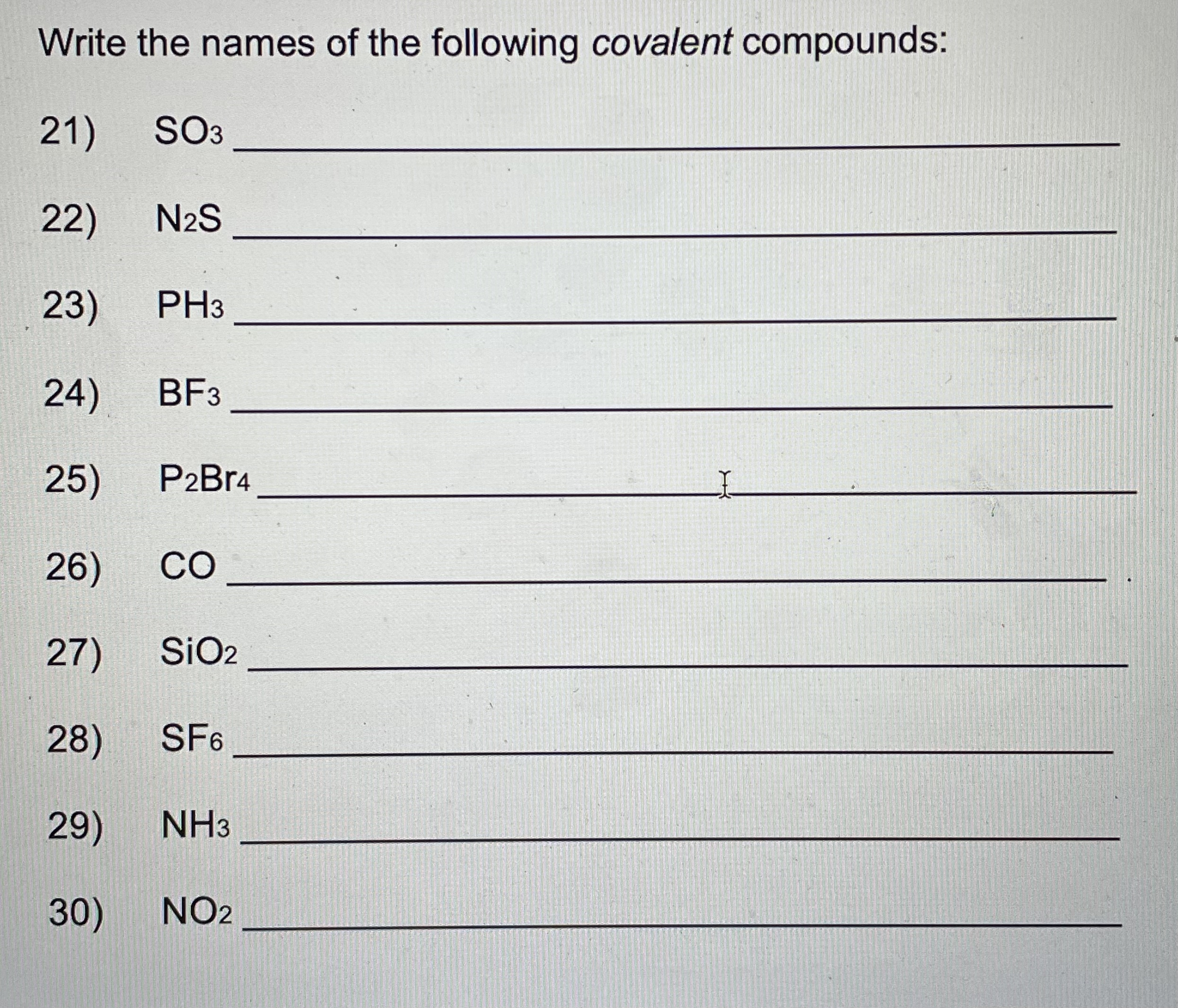
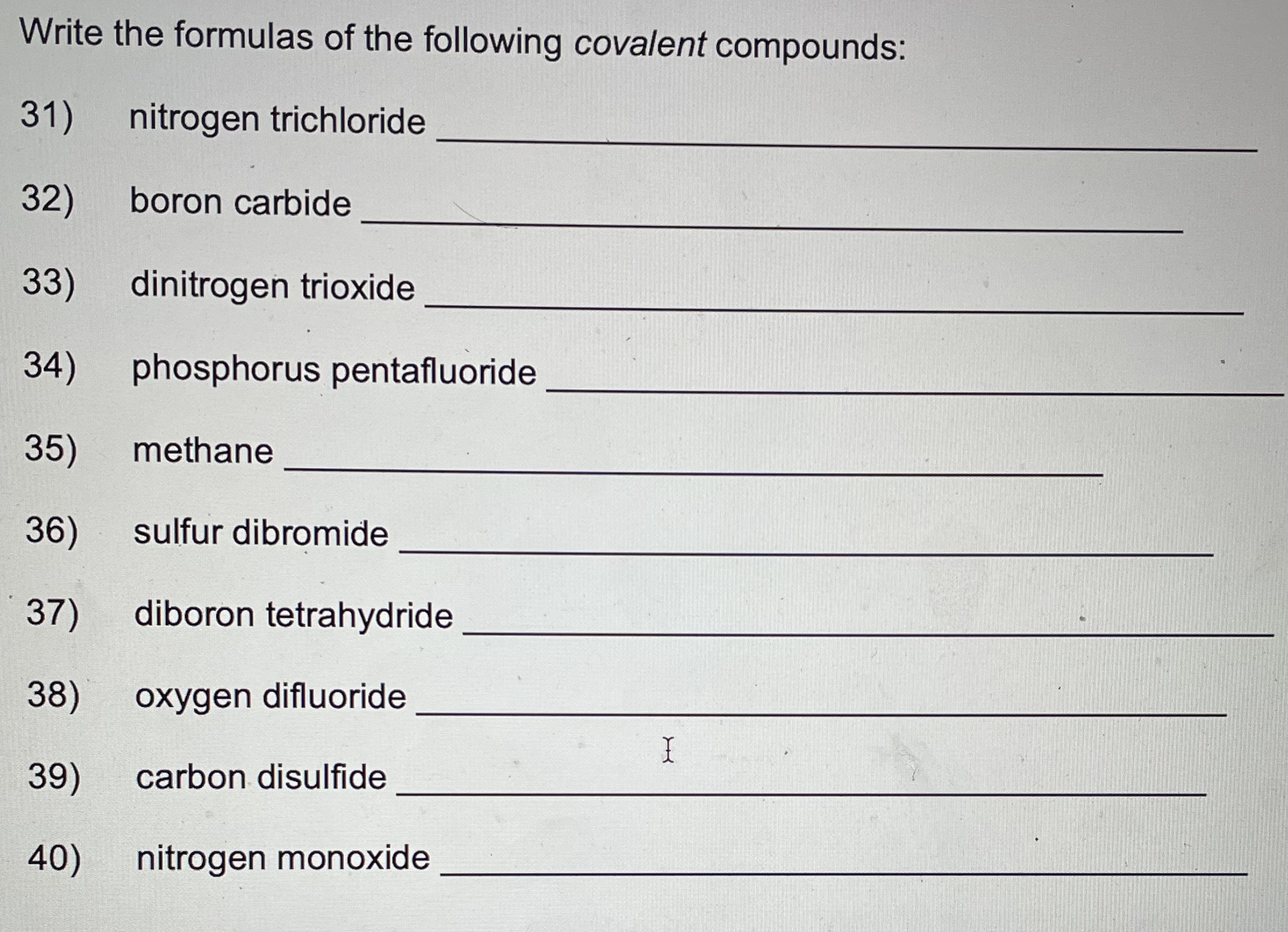
Write the formulas for the following ionic compounds: 6) potassium iodide 7) magnesium oxide 8) aluminum chloride 9) sodium nitrate 10) calcium carbonate 11) lithium sulfate , 12) beryllium phosphide 13) magnesium hydroxide 14) sodium phosphate 1 15) aluminum carbonate 16) calcium chloride 17) sodium cyanide 18) aluminum oxide 19) magnesium acetate 20) ammonium chloride Naming Chemical Compounds Worksheet Name the following ionic compounds: 1 ) NaBr 2) CaO 3) LizS 4) MgBr2 5) Be(OH)2Write the names of the following covalent compounds: 21) SO3 22) N2S 23 ) PH3 24) BF3 25) P2 Br4 26) CO 27) SiO2 28 ) SF6 29) NH3 30) NO2Write the formulas of the following covalent compounds: 31) nitrogen trichloride 32) boron carbide 33) dinitrogen trioxide 34) phosphorus pentafluoride 35) methane 36) sulfur dibromide 37 ) diboron tetrahydride 38) oxygen difluoride 39) carbon disulfide 40) nitrogen monoxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


