Question: Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106.
Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106.
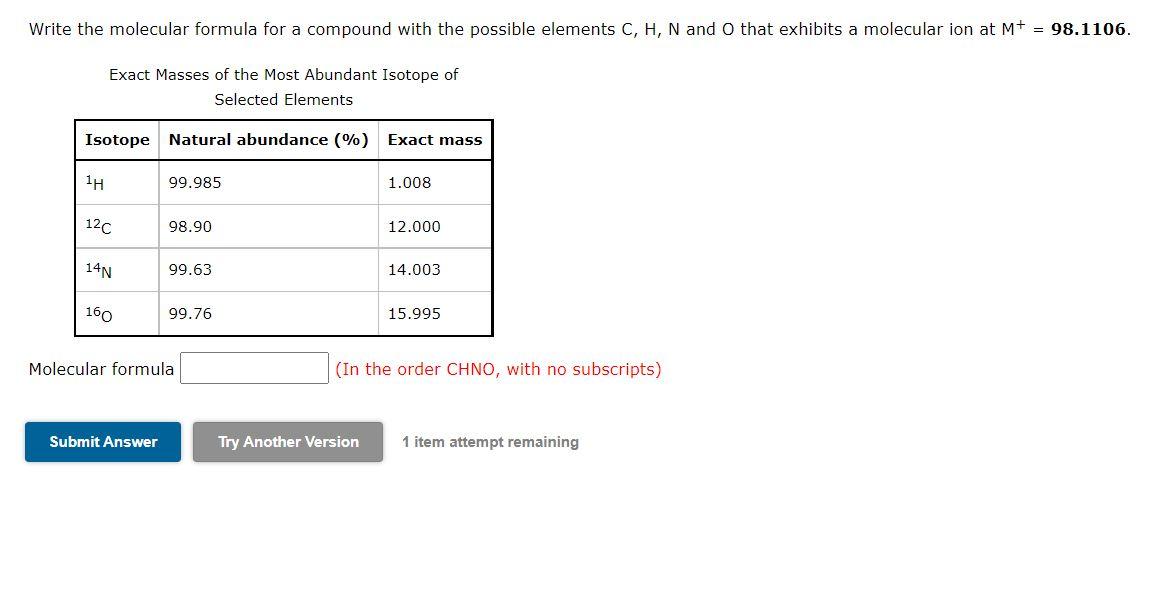
Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts) Submit Answer Try Another Version 1 item attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


