Question: Write the molecular formula for a compound with the possible elements C, H, N and o that exhibits a molecular ion at M+ = 128.0632.
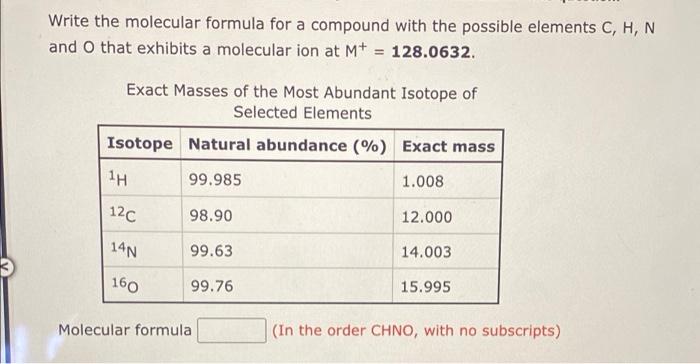
Write the molecular formula for a compound with the possible elements C, H, N and o that exhibits a molecular ion at M+ = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 120 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


