Question: Written Solution Please Problem 2: Raoult's Law flash, From Problem 6.41 in Felder and Rousseau ( 3rd Edition) A liquid stream consisting of 12.5 mole
Written Solution Please
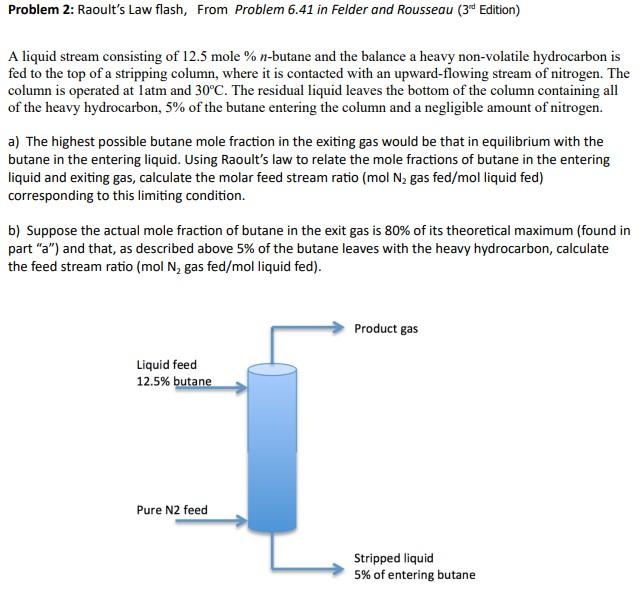
Problem 2: Raoult's Law flash, From Problem 6.41 in Felder and Rousseau ( 3rd Edition) A liquid stream consisting of 12.5 mole \% n-butane and the balance a heavy non-volatile hydrocarbon is fed to the top of a stripping column, where it is contacted with an upward-flowing stream of nitrogen. The column is operated at latm and 30C. The residual liquid leaves the bottom of the column containing all of the heavy hydrocarbon, 5% of the butane entering the column and a negligible amount of nitrogen. a) The highest possible butane mole fraction in the exiting gas would be that in equilibrium with the butane in the entering liquid. Using Raoult's law to relate the mole fractions of butane in the entering liquid and exiting gas, calculate the molar feed stream ratio ( molN2 gas fed/mol liquid fed) corresponding to this limiting condition. b) Suppose the actual mole fraction of butane in the exit gas is 80% of its theoretical maximum (found in part "a") and that, as described above 5% of the butane leaves with the heavy hydrocarbon, calculate the feed stream ratio ( molN2 gas fed/mol liquid fed)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


