Question: wrong answer help please MISSED THIS? Read Section 15.4 (Pages 642 - 649) ; Watch KCV 15.4. IWE 15.4. The decomposition of SO2Cl2 is first

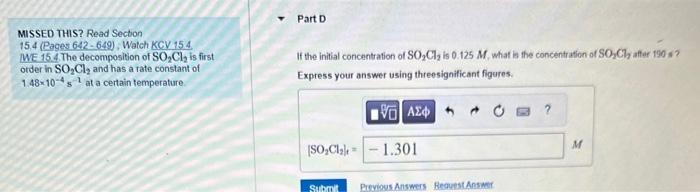
MISSED THIS? Read Section 15.4 (Pages 642 - 649) ; Watch KCV 15.4. IWE 15.4. The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.48104s1 at a certain temperature. MISSED THIS? Read Sectoon 15.4 (Pages 642 - 649), Watch KCV 154. We 15.4. The decomposition of SO2Cl2 is first If the initial concentration of SO2Cl2 is 0.125M, what is the concentr ation of SO2Cl2 affer 190 s ? order in SO2Cl2 and has a rate constant of 1.48104s1 at a certain temperature Express your answer using threesignificant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


