Question: Yield calculation (show all calculations, including the dimensional analysis and units in all quantities in equations, with correct number of significant figures) Moles of acetophenone:
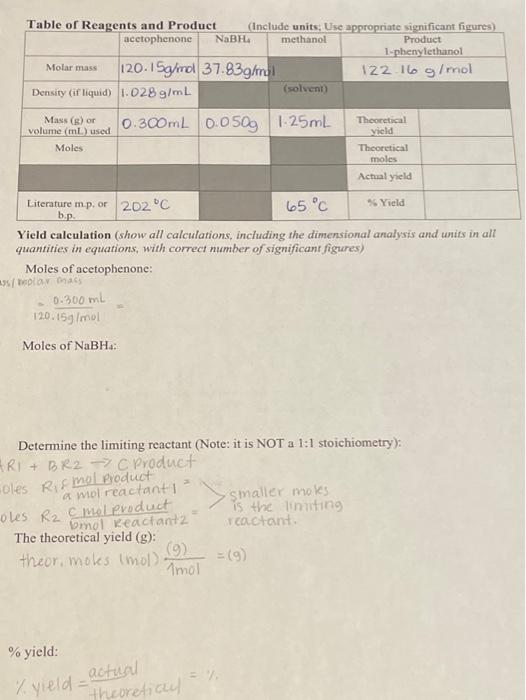
Yield calculation (show all calculations, including the dimensional analysis and units in all quantities in equations, with correct number of significant figures) Moles of acetophenone: =120.15g/mul0.300mL= Moles of NaBH4 : Determine the limiting reactant (Note: it is NOT a 1:1 stoichiometry): RI1+BX2 Croduct Qles Rif mol product The theoretical yield (g) : theor. moks ( mol )Amol(9)=(9) % yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


