Question: You are given a 5.2 ml copper sample that has a density of 8.96g/ml. what mass of copper do you have? and you are given
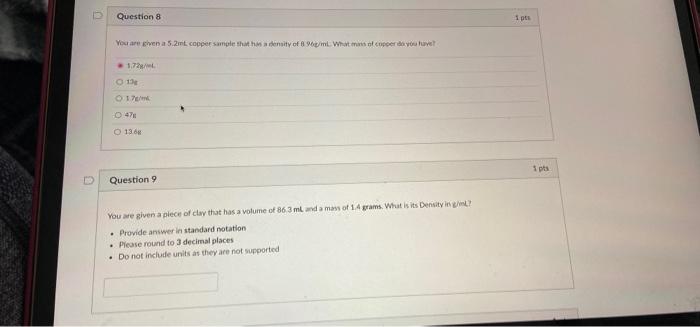
12 Question 8 i pts You are given a 5.2mE copper sample that ensity of W/m. What cuper de volve! 1.72 134 17 O 136 1 pts Question You are given a piece of clay that has a volume of 863 mlada mays of 14 grams. What is its Densityin! Provide answer in standard notation Please round to 3 decimal places Do not include units as they are not supported
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


