Question: You are to develop a spreadsheet to carry out material and energy balances on an isothermal reactor being used to produce methyl acetate. This is

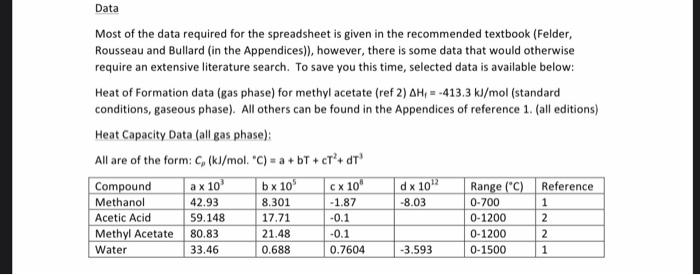
You are to develop a spreadsheet to carry out material and energy balances on an isothermal reactor being used to produce methyl acetate. This is formed by the gas phase reaction between methanol and acetic acid as per the reaction below: CH,OH + CH2COOH CH,COOCH3 +H20 The reaction occurs in the gaseous phase with a reactor temperature of between 300 and 400C. Prior to reaction, the mixture of gaseous methanol and acetic acid is heated from 150C to the reaction temperature in a heat exchanger, before entering the reactor. The reactor is maintained at the required temperature to ensure that methanol conversion is maintained. The spreadsheet that you must develop for the assignment should allow a user to change the input flowrates of reactants as well as the temperature of the isothermal reactor and the conversion of one of the reactants (or extent of reaction) and calculate the output from the reactor. It should also allow the user to input the relevant physical properties data for the reactants and products and using the input and calculated flowrates the energy added or removed from the reactor to maintain isothermal function, as well as the energy required in the heat exchanger prior to the reactor to raise the temperature of the reactants from 150C to the reaction temperature. Once you have developed your spreadsheet, you need to determine the following: a. For an input of 150 mol/h of methanol, 100 mol/h acetic acid with a methanol conversion of 40%, the energy input required from the heat exchanger to raise the input stream temperature from 150C to 350C, the reactor output molar flowrate and mole fractions, and the energy added or removed from the reactor for isothermal operation at 350'C. b. For an input of 100 mol/h of methanol, 150 mol/h acetic acid with a methanol conversion of 30%, the energy input required from the heat exchanger to raise the input stream temperature from 150C to 300C, the reactor output molar flowrate and mole fractions, and the energy added or removed from the reactor for isothermal operation at 300C. c. For an input of 200 mol/h of methanol, 150 mol/h acetic acid with a methanol conversion of 45%, the energy input required from the heat exchanger to raise the input stream temperature from 150C to 400C, the reactor output molar flowrate and mole fractions, and the energy added or removed from the reactor for isothermal operation at 400C. Data Most of the data required for the spreadsheet is given in the recommended textbook (Felder, Rousseau and Bullard (in the Appendices)), however, there is some data that would otherwise require an extensive literature search. To save you this time, selected data is available below: Heat of Formation data (gas phase) for methyl acetate (ref 2) AH,- -413.3 kJ/mol (standard conditions, gaseous phase). All others can be found in the Appendices of reference 1. (all editions) Heat Capacity Data (all gas phase): All are of the form: C,(kJ/mol. "C) = a + bT + cr + OT! Compound a x 10 b x 10 CX 10 d x 10 Range("C) Reference Methanol 42.93 8.301 -1.87 -8.03 0-700 Acetic Acid 59.148 17.71 -0.1 0-1200 2 Methyl Acetate 80.83 21.48 -0.1 0-1200 2 Water 33.46 0.688 0.7604 -3.593 0-1500 1 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


