Question: YOU CAN JUST SOLVE QUESTION #1, I HAVE SOLVED THE REST BUT I POSTED IT FOR YOU TO MAKE SURE THEY ARE RIGHT, SO PLZ
YOU CAN JUST SOLVE QUESTION #1, I HAVE SOLVED THE REST BUT I POSTED IT FOR YOU TO MAKE SURE THEY ARE RIGHT, SO PLZ LEAVE AN OPINION IN EACH AND TELL ME IF THEY ARE RIGH OR WRONG, THANKS!
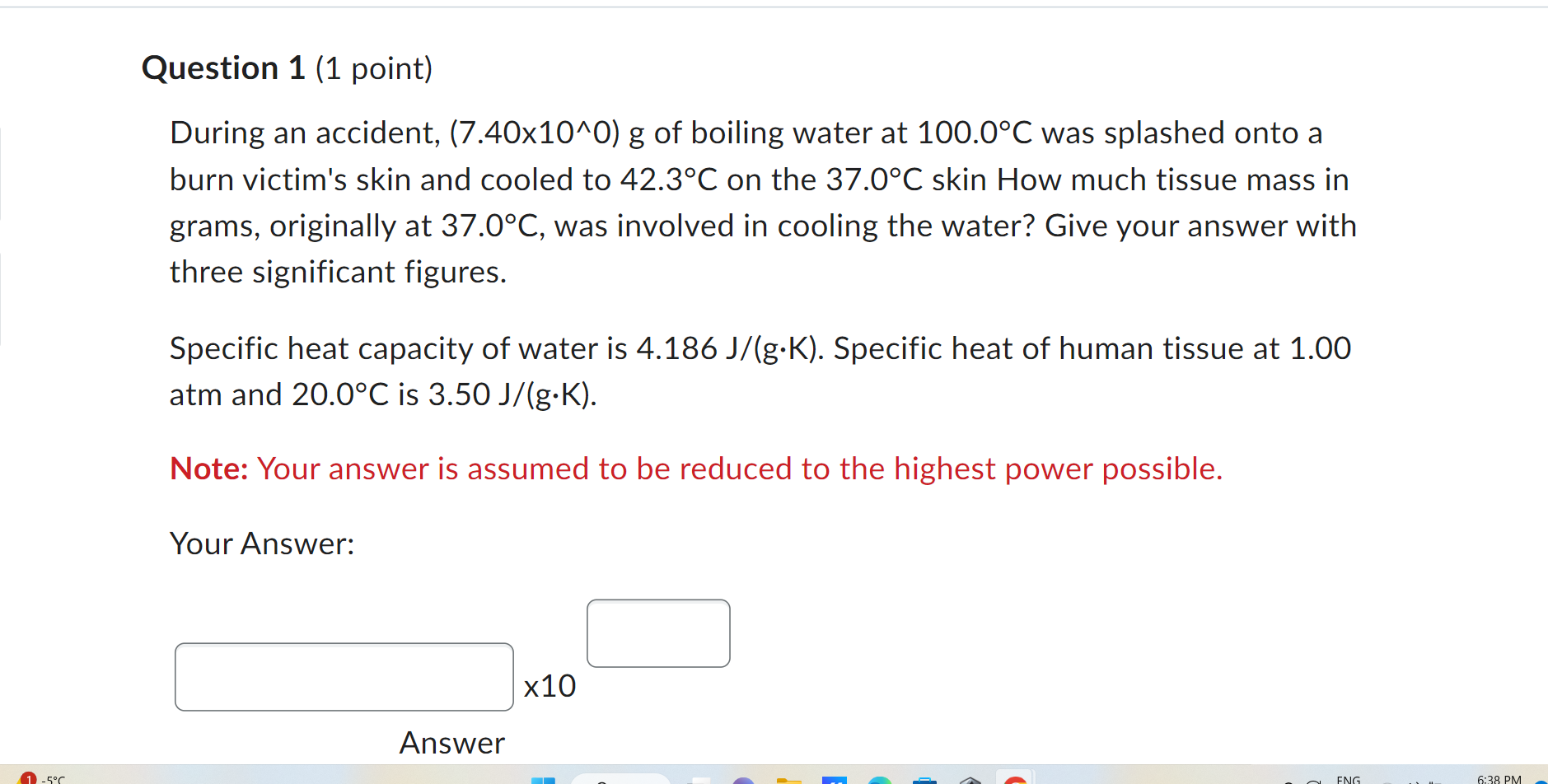
Question 1 (1 point) During an accident, (7.40x10^0) g of boiling water at 100.0 C was splashed onto a burn victim's skin and cooled to 42.3C on the 37.0 C skin How much tissue mass in grams, originally at 37.0 C, was involved in cooling the water? Give your answer with three significant figures. Specific heat capacity of water is 4.186 J/(g.K). Specific heat of human tissue at 1.00 atm and 20.0 C is 3.50 J/(g.K). Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


