Question: You can use this formula for x and y PROBLEM 6.1 CONSTRUCTION OF EQUILIBRIUM DIAGRAMS From the data on the solubility of NH in water
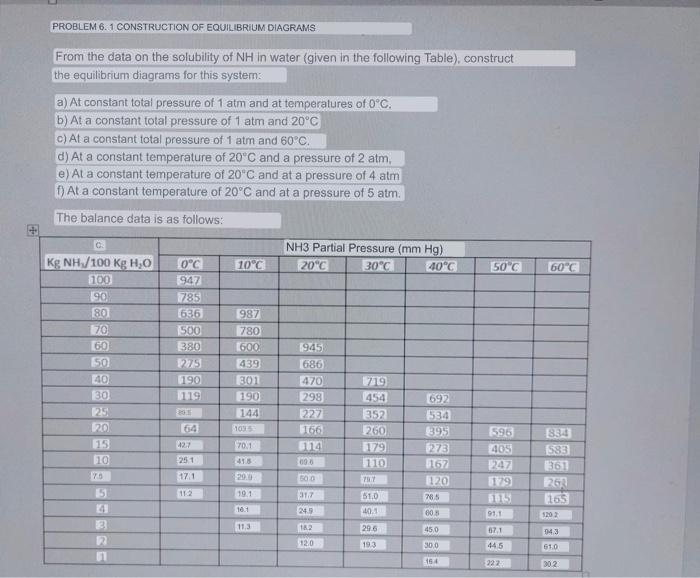
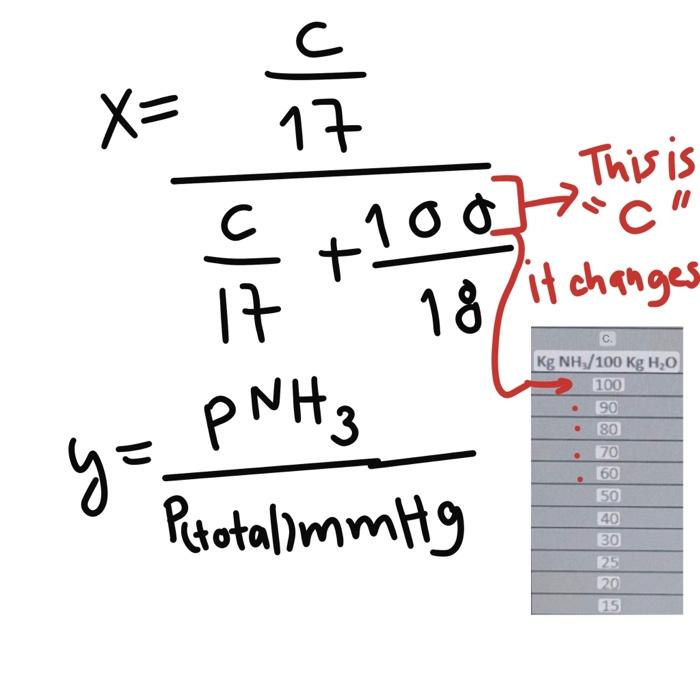
PROBLEM 6.1 CONSTRUCTION OF EQUILIBRIUM DIAGRAMS From the data on the solubility of NH in water (given in the following Table), construct the equilibrium diagrams for this system: a) At constant total pressure of 1atm and at temperatures of 0C. b) At a constant total pressure of 1atm and 20C c) At a constant total pressure of 1 atm and 60C. d) At a constant temperature of 20C and a pressure of 2atm, e) At a constant temperature of 20C and at a pressure of 4atm f) At a constant temperature of 20C and at a pressure of 5atm. The balance data is as follows: y=PtotalmmmHgPNH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


