Question: You have a 1.153g sample of an unknown solid acid, HA dissolved in enough water to make 20.00mL of solution. HA reacts with KOH(aq) according
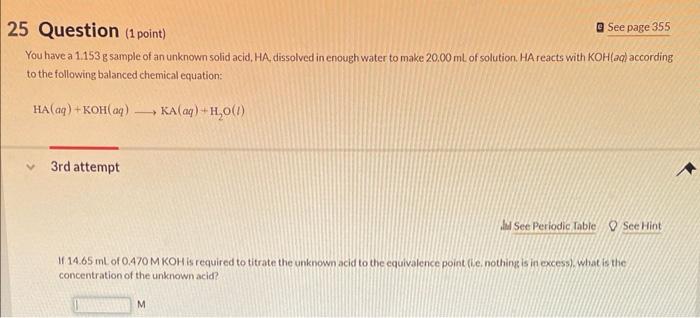
You have a 1.153g sample of an unknown solid acid, HA dissolved in enough water to make 20.00mL of solution. HA reacts with KOH(aq) according to the following balanced chemical equation: HA(aq)+KOH(aq)KA(aq)+H2O(l) 3rd attempt If 14.65mL of 0.470MKOH is required to titrate the unknown acid to the equivalence point (i.e. nothing is in excess), what is the concentration of the unknown acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


