Question: You have a reaction with liquid phase reactants and products. The reaction is catalyzed by porous spherical catalyst and has the following mechanism: A+SASASB(l)+CS(RLS)CSC+S a)
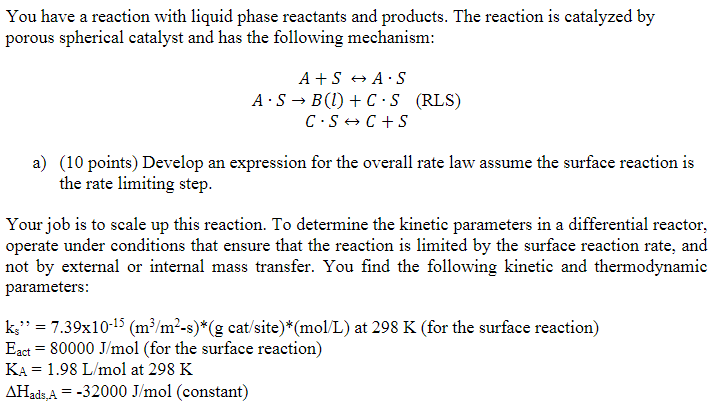


You have a reaction with liquid phase reactants and products. The reaction is catalyzed by porous spherical catalyst and has the following mechanism: A+SASASB(l)+CS(RLS)CSC+S a) (10 points) Develop an expression for the overall rate law assume the surface reaction is the rate limiting step. Your job is to scale up this reaction. To determine the kinetic parameters in a differential reactor, operate under conditions that ensure that the reaction is limited by the surface reaction rate, and not by external or internal mass transfer. You find the following kinetic and thermodynamic parameters: ks=7.391015(m3/m2s)(gcat/site)(mol/L) at 298K (for the surface reaction) Eact=80000J/mol (for the surface reaction) KAA=1.98L/mol at 298K Hads,A=32000J/mol (constant) KB=0.0005L/molat298KHads,B=9000J/mol(constant)KC=3.15L/molat298KHads,C=28000J/mol(constant)R=8.3145J/(molK)Ct=108sites/(gcat) b) (10 points) You plan on creating an isothermal process with T=800K. Calculate the rate constant k(k=ksKACt), and the equilibrium adsorption constants KA,KB and KC at the new operating temperature. How can the rate equation from part (b) be simplified for this new operating temperature? For scale up, you develop a process in a packed bed reactor with the porous spherical catalyst pellets operating at a high temperature. The pressure drop is negligible. You have the following additional information: T=800KCAb,0=0.2mol/Lv0=3.87104m3/sDpipe=0.5m=0.4dp=0.01mDe=1.82108m2/sSa=500m2/gc=2.8106g/m3v=1.53108m2/sDAB=2.0108m2/s c) (5 points) Calculate the Thiele modulus for these operating conditions d) (5 points) Calculate the internal effectiveness factor, , for these operating conditions e) (10 points) Calculate the mass transfer coefficient, kc, for these operating conditions f) ( 5 points) Calculate the overall effectiveness factor, , for these operating conditions g) (5 points) Calculate the mass of catalyst you would need to achieve 98% conversion h) (10 points) Is the reaction limited by external mass transfer, internal mass transfer, or the surface reaction rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


