Question: You may want to reference (Pages 245 - 246) Section 7.8 while completing this problem. Iron(III) oxide reacts with carbon to give iron and carbon

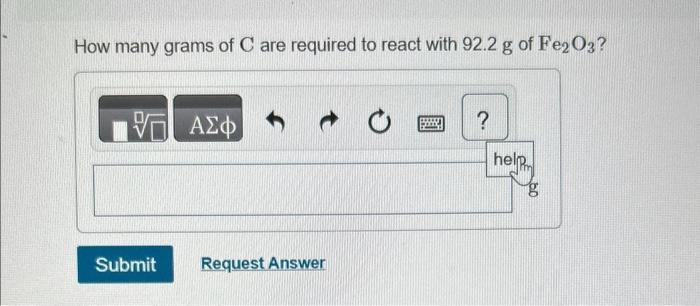
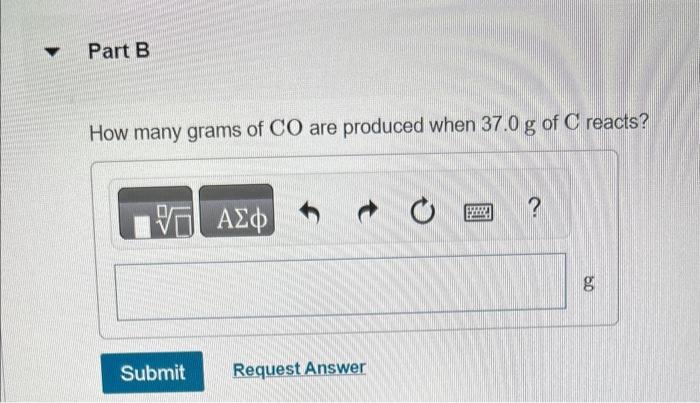
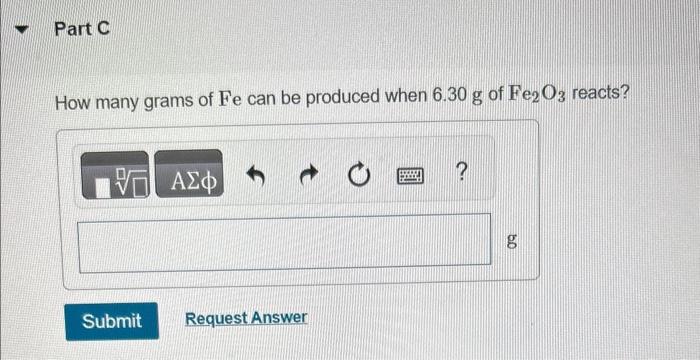
You may want to reference (Pages 245 - 246) Section 7.8 while completing this problem. Iron(III) oxide reacts with carbon to give iron and carbon monoxide: Fe2O3(s)+3C(s)2Fe(s)+3CO(g) How many grams of C are required to react with 92.2g of Fe2O3 ? How many grams of CO are produced when 37.0g of C reacts? How many grams of Fe can be produced when 6.30g of Fe2O3 reacts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


