Question: A solution of benzene (CH) and toluene (C7Hs) is 29.0 benzene by mass. At 25C the vapor pressures of pure benzene and pure toluene
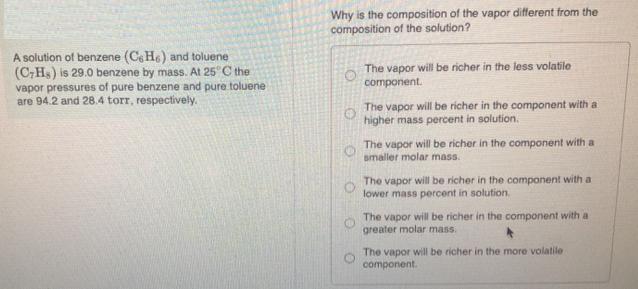
A solution of benzene (CH) and toluene (C7Hs) is 29.0 benzene by mass. At 25C the vapor pressures of pure benzene and pure toluene are 94.2 and 28.4 torr, respectively. Why is the composition of the vapor different from the composition of the solution? O O O The vapor will be richer in the less volatile component. The vapor will be richer in the component with a higher mass percent in solution. The vapor will be richer in the component with a smaller molar mass. The vapor will be richer in the component with a lower mass percent in solution. The vapor will be richer in the component with a greater molar mass. The vapor will be richer in the more volatile component.
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided b... View full answer

Get step-by-step solutions from verified subject matter experts


