Question: You prepare 1.0 L of a binary solvent solution with a 10.0x10 -6 M formal concentration of CoCl2. You prepare the solution at 25.0 C
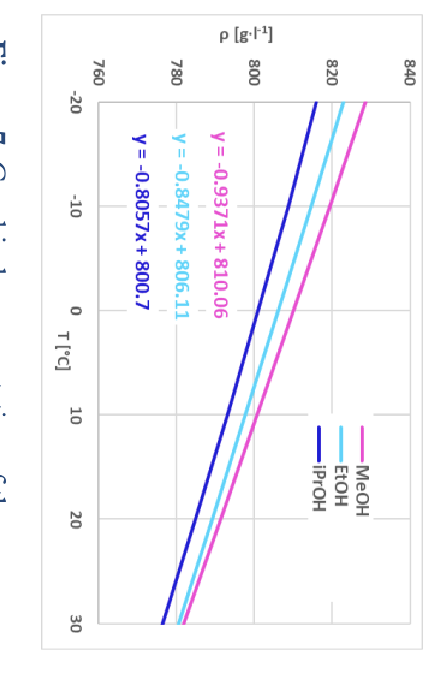
You prepare 1.0 L of a binary solvent solution with a 10.0x10-6 M formal concentration of CoCl2. You prepare the solution at 25.0 C with 500.0 mL of methanol (R1OH) and 500.0 mL of ethanol (R2OH). Due to the temperature dependence of the solvent densities, the formal concentration of CoCl2 in this solution would also change with temperature. In this experiment it is necessary to account for this temperature dependence. Figure 7 and the Background section for Milestone 2 should be consulted for answering the following questions. For these questions, setup up a spreadsheet to perform these calculations as this is something you will employ throughout this entire experiment. o Calculate and report what the volume of 500.0 mL of methanol, prepared at 25.0 C, would be at -10 C. o Calculate and report what the volume of 500.0 mL of ethanol, prepared at 25.0 C, would be at -10 C. o Assuming that a binary mixture of ethanol and methanol behaves ideally, calculate and report what the volume of the 1.0 L binary solvent solution, prepared at 25.0 C with 500.0 mL of ethanol and 500.0 mL of methanol, would be at -10 C. o Calculate and report what the formal concentration of CoCl2 for the solution, prepared at 25.0 C, would be at -10 C. o Calculate and report the percent change in formal concentration, relative to the solution prepared at 25.0 C, when it is cooled to -10 C.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


