Question: You prepare a buffer solution from 10.0mL of 0.100M MOPS (3-morpholinopropane-1-sulfonic acid) and 10.0mL of 0.089MNaOH. Next, you add 1.00mL of 4.27105M lidocaine to this
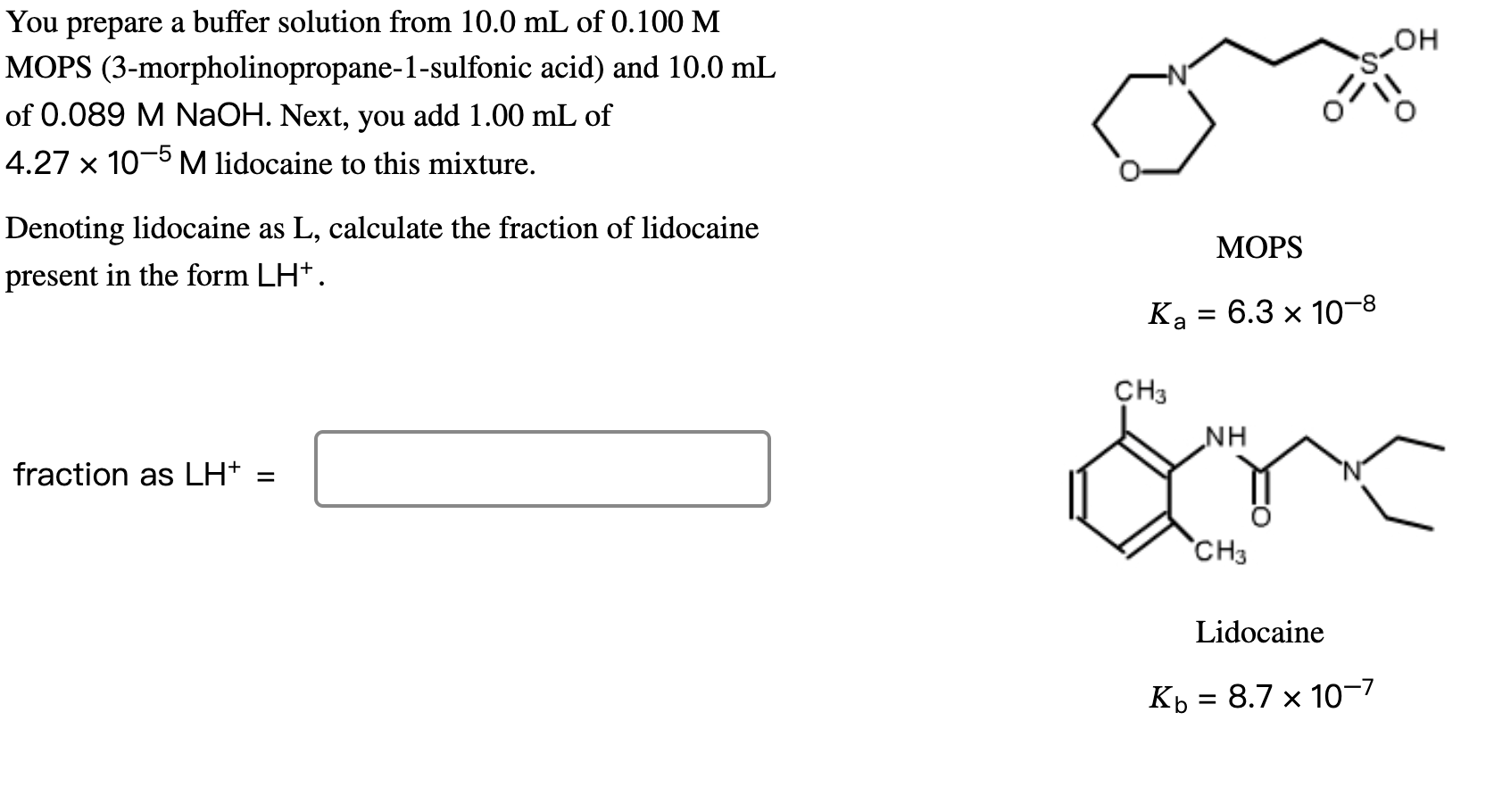
You prepare a buffer solution from 10.0mL of 0.100M MOPS (3-morpholinopropane-1-sulfonic acid) and 10.0mL of 0.089MNaOH. Next, you add 1.00mL of 4.27105M lidocaine to this mixture. Denoting lidocaine as L, calculate the fraction of lidocaine MOPS present in the form LH+. Ka=6.3108 fraction as LH+= Lidocaine Kb=8.7107
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


