Question: You will use the values provided by the previous zero-point quiz to complete this assignment. For the distillation of an isopropanol/isobutanol (iPrOH/iBuOH) mixture, you were
You will use the values provided by the previous zero-point quiz to complete this assignment. For the distillation of an isopropanol/isobutanol (iPrOH/iBuOH) mixture, you were provided:
***NOTE: THESE ARE THE VALUES PROVIDED ON THE PREVIOUS QUIZ
initial mixture is 66.8% isobutanol
the temperature of the distillation is 85.7 degrees celsius
the composition of the mixture to be distilled (volume percent isobutanol in isopropanol)
the temperature at which the initial distillate came over.
A phase diagram for this system is provided as a PDF:
iPrOH_iBuOH_phase_diagram.pdf
The working assumption is that you will print out the diagram; work on it (I STRONGLY suggest using pencil so you can correct any mistakes), and then scan and upload it as well as your other work (calculations; stated number of theoretical plates).
If you do not have a working printer, you can plot a version on graph paper, or place paper on your monitor and trace it, etc. Let your TA know this is what you're doing, so they can check and make sure it's legible. As long as it's clear how you determined theoretical plates, the accuracy of your reproduction of the graph won't matter.
Additional useful information:
The molecular weight of isopropanol is 60.10 g/mol
The density of isopropanol is 0.786 g/mL
The boiling point of isopropanol is 82.6 C
The molecular weight of isobutanol is 74.12 g/mol
The density of isobutanol is 0.802 g/mL
The boiling point of isobutanol is 107.9 C
You will use this data, and the diagram, to determine the number of theoretical plates the distillation apparatus provided.
To do this, you will need to know the composition of the initial mixture, and the initial distillate, in mole fraction. Mole fraction is mole percent divided by 100.
For the initial mixture to be distilled, you will convert vol% iBuOH to mole fraction iBuOH. Hint: start your calculation assuming you have any convenient volume of solution (e.g. 100 mL) and go from there. You must show your calculations.
For the initial distillate, use the phase diagram and interpolate the mole fraction by tracing from the temperature on the y-axis, to the intercept with the liquid phase (lower) curve, down to the x-axis. Show this by drawing these lines on the graph.
Then, on the graph, trace evaporate/condense cycles (theoretical plates) from the initial mixture composition until you meet or pass the initial distillate composition. Report the number of theoretical plates that the distillation apparatus provided, e.g. "about 2", "between 2 and 3", "not quite 3" etc. Your description must match the work you show on the graph.
please use this attatched photo as a template for the phase diagram. the "y" axis is temperaure.
Thank you.
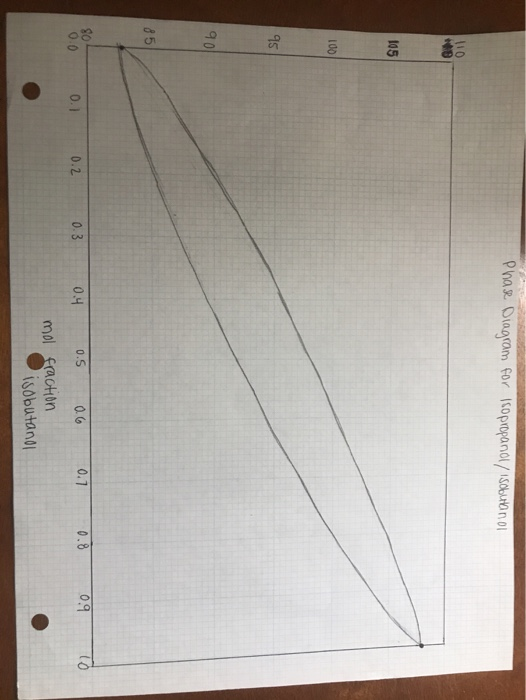
Phase Diagram for Isopropanol/isabethnol 110 10 105 100 95 90 85 SO 0.0 0.2 0.3 0.5 0.1 0.4 0.6 0.8 0.1 0.9 mol fraction isobutano Phase Diagram for Isopropanol/isabethnol 110 10 105 100 95 90 85 SO 0.0 0.2 0.3 0.5 0.1 0.4 0.6 0.8 0.1 0.9 mol fraction isobutano
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


