Question: Your answer is partially correct. Aerobic organisms transfer electrons from reduced fuel molecules to O2, forming H20. Some anaerobic organisms use nitrate (NO3 ) as
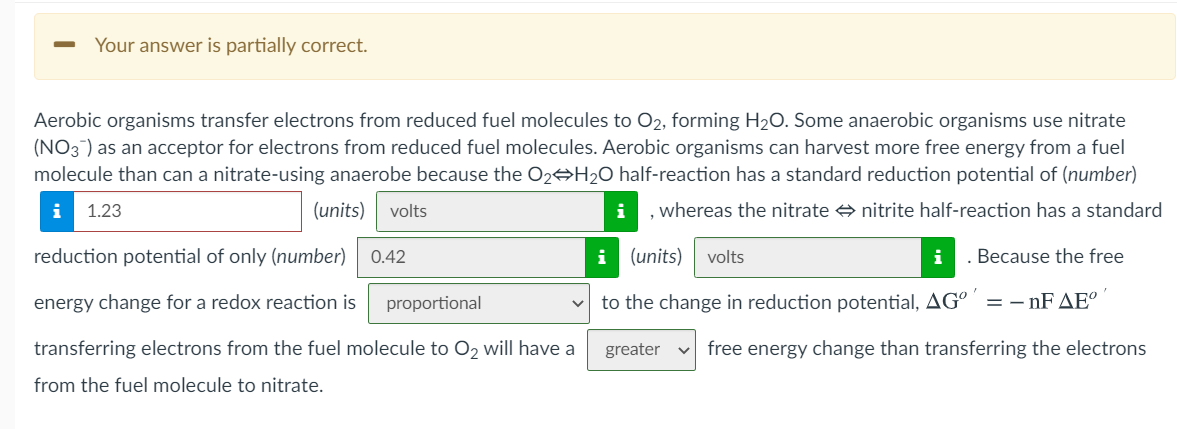
Your answer is partially correct. Aerobic organisms transfer electrons from reduced fuel molecules to O2, forming H20. Some anaerobic organisms use nitrate (NO3 ) as an acceptor for electrons from reduced fuel molecules. Aerobic organisms can harvest more free energy from a fuel molecule than can a nitrate-using anaerobe because the 02 H2O half-reaction has a standard reduction potential of (number) i 1.23 (units) volts , whereas the nitrate nitrite half-reaction has a standard reduction potential of only (number) 0.42 i (units) volts Because the free energy change for a redox reaction is proportional to the change in reduction potential, AG = - nF AE greater vfree energy change than transferring the electrons transferring electrons from the fuel molecule to O2 will have a from the fuel molecule to nitrate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


