Question: Your instructor will provide a method of compiling team data for the average rate of the reaction across all concentrations. Record (either by hand or
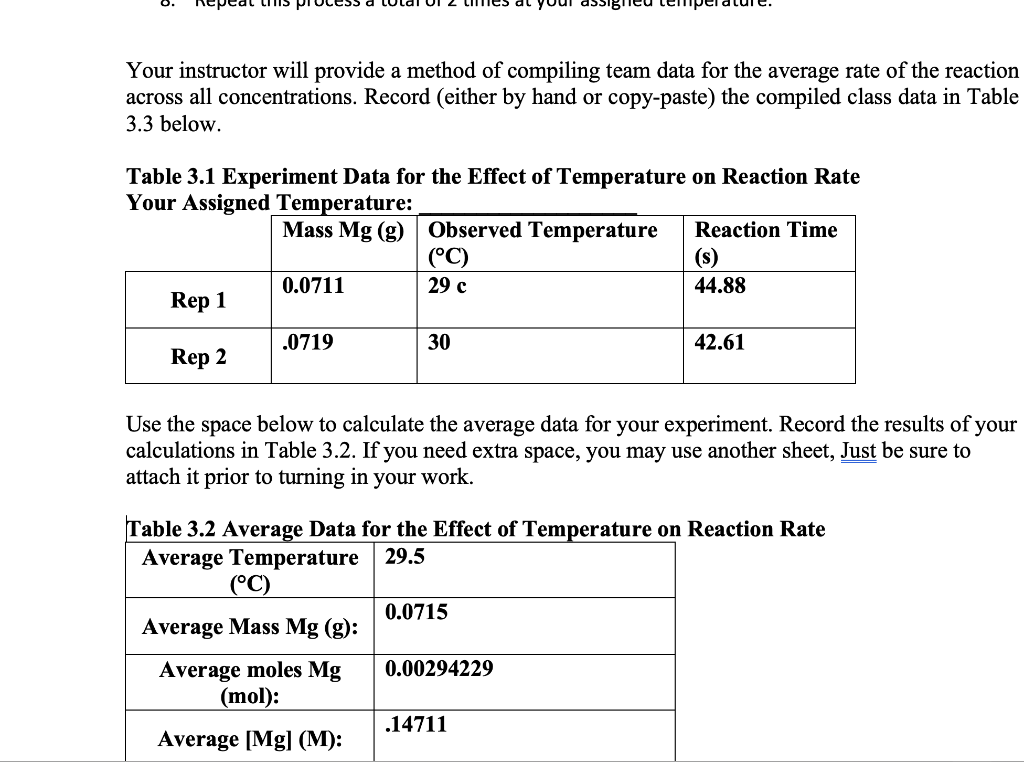
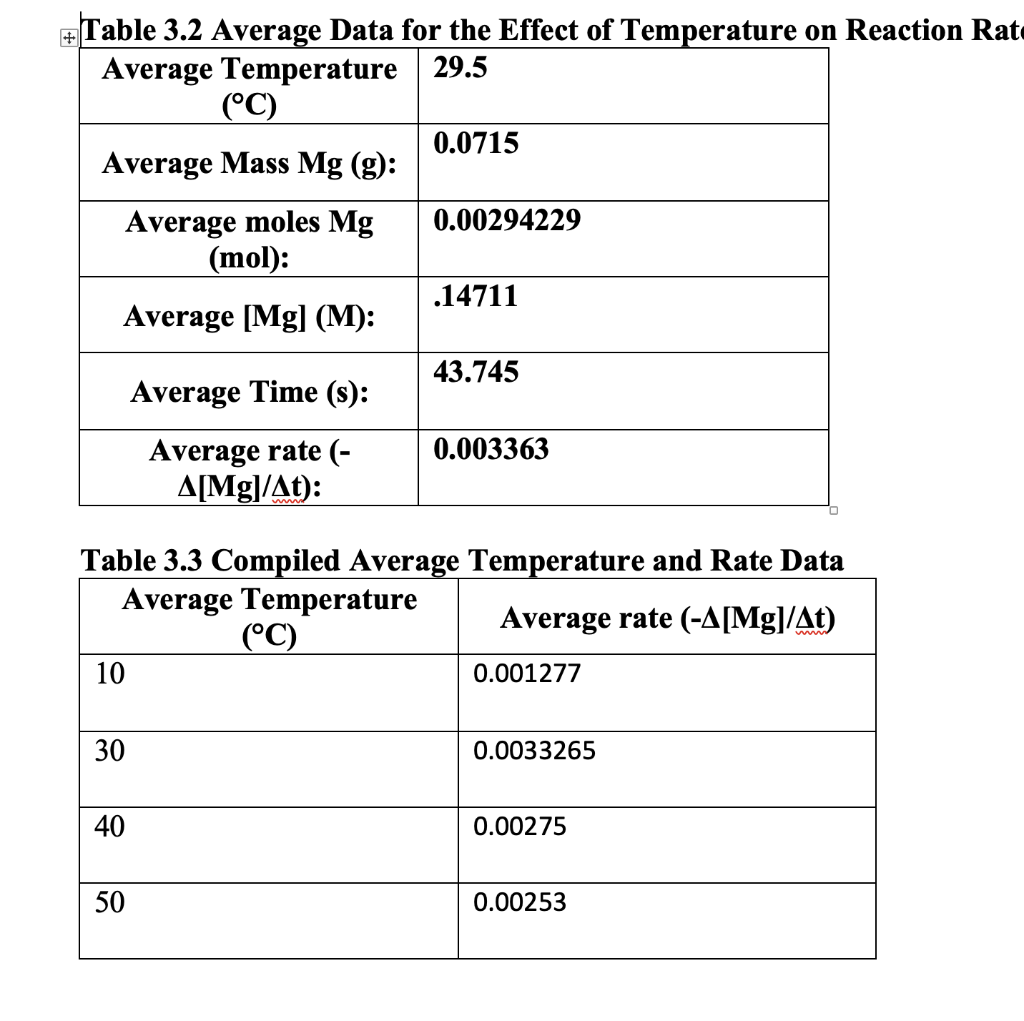


Your instructor will provide a method of compiling team data for the average rate of the reaction across all concentrations. Record (either by hand or copy-paste) the compiled class data in Table 3.3 below. Table 3.1 Experiment Data for the Effect of Temperature on Reaction Rate Your Assigned Temperature: Use the space below to calculate the average data for your experiment. Record the results of your calculations in Table 3.2. If you need extra space, you may use another sheet, Just be sure to attach it prior to turning in your work. Table 3.2 Average Data for the Effect of Temperature on Reaction Rate Table 3.2 Average Data for the Effect of Temperature on Reaction Rat \begin{tabular}{|c|l|} \hline AverageTemperature(C) & 29.5 \\ \hline AverageMassMg(g): & 0.0715 \\ \hline AveragemolesMg(mol): & 0.00294229 \\ \hline Average [Mg](M): & .14711 \\ \hline Average Time (s): & 43.745 \\ \hline Averagerate([Mg]/t): & 0.003363 \\ \hline \end{tabular} Table 3.3 Compiled Average Temperature and Rate Data \begin{tabular}{|l|l|} \hline \multicolumn{1}{|c|}{AverageTemperature(C)} & \multicolumn{1}{|c|}{ Average rate ([Mg]/t)} \\ \hline 10 & 0.001277 \\ \hline 30 & 0.0033265 \\ \hline 40 & 0.00275 \\ \hline 50 & 0.00253 \\ \hline \end{tabular} Calculate the value of k for your assigned temperature in the rate expression Show your calculations and explain your answer below. If you need extra space, you may use another sheet, Just be sure to attach it prior to turning in your work. rate=k[Mg]x[HCl]y Are the results in agreement with your hypothesis? Why or why not? Show your calculations and explain your answer below. Using the compiled class data, calculate how much the value of k changes for every 10C increase in temperature between 10C40C. Is there a pattern? Show your calculations and explain your answer below. If you need extra space, you may use another sheet, Just be sure to attach it prior to turning in your work. Lab Report Provide a brief report describing any trends you observed in the compiled class data for each experiment. Be sure to highlight the effects of the initial reactant concentrations and temperature on the reaction rate and the rate constant k. Also, answer the following questions in your report: What is the rate law for this reaction and how do you know? What is the order of the reaction? Consider the state of the Mg when you added it to the acid. What if you used magnesium chips or magnesium powder instead? Could the state of the Mg at the start of the reaction effect the rate of the reaction? Explain. Did you observe in deviation in the value of k between experiments? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


