Question: Your task is to provide a procedure for purifying a desired organic compound by removing an undesired impurity, using the technique of extraction. The scenario
Your task is to provide a procedure for purifying a desired organic compound by removing an undesired impurity, using the technique of extraction.
The scenario starts with a solution of the desired compound plus undesired impurity (Which is below, in the picture) in the immiscible organic solvent of your choice. The solvent choice is not critical. However, if you take its density into consideration, your choice of solvent could make the procedure shorter.
For reagents, you can assume that you have the following at your disposal:
- 5% aqueous HCl
- 5% aqueous NaOH
- saturated sodium bicarbonate
- saturated sodium carbonate
- saturated aqueous sodium chloride
- sodium sulfate
- extra immiscible organic solvent of your choice (e.g. if your initial mixture is dissolved in diethyl ether, extra diethyl ether will be available)
Any laboratory glassware and equipment needed for the procedure (e.g. separatory funnel; rotary evaporator) would be available as well. (put it into procedure)
Provide a written procedure for purifying the desired compound, starting from the impure organic solution and ending with the pure desired compound. The procedure should be detailed enough that an undergraduate student could follow the procedure with ease.
- They would need to be told what steps to perform, but it will be understood that they know how to perform the techniques involved. For example, "extract solution X with solution Y" doesn't require explaining how to grip a separatory funnel.
- They would need to be told whether the organic layer is the upper or the lower layer.
- They also need to know what hazards are involved and what precautions to take. However, for this exercise, assume that the SDSs for all the compounds and reagents have already been reviewed with the student--you don't have to look up SDSs for this exercise.
Picture below: on the left is the desired compound, on the right is the undesired impurity.
**Working on a microscale (100 mg solute mixture in 2 mL organic solvent)
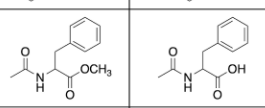
Please help, thank you!
OCH, . OH IZ IZ 0 o
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


