Question: A 10 mol% isopropanol-in-water mixture at its bubble point is to be distilled at 1 atm to produce a distillate containing 67.5 mol% isopropanol, with
A 10 mol% isopropanol-in-water mixture at its bubble point is to be distilled at 1 atm to produce a distillate containing 67.5 mol% isopropanol, with 98% recovery. At a reflux ratio L=D of 1.5 times the minimum, how many theoretical stages will be required:
(a) if a partial reboiler is used?
(b) if no reboiler is used and saturated steam at 101 kPa is introduced below the bottom plate?
(c) How many stages are required at total reflux?
Vapor–liquid data in mole-fraction isopropanol at 101 kPa are:
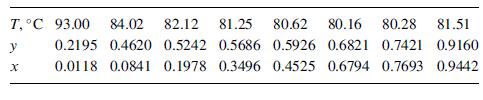
T,C 93.00 y 84.02 82.12 81.25 80.62 80.16 80.28 81.51 0.2195 0.4620 0.5242 0.5686 0.5926 0.6821 0.7421 0.9160 0.0118 0.0841 0.1978 0.3496 0.4525 0.6794 0.7693 0.9442 X
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
To answer this question we will need to use the McCabeThiele method to determine the number of theor... View full answer

Get step-by-step solutions from verified subject matter experts


