A bottle of pure n-heptane is accidentally poured into a drum of pure toluene in a laboratory.
Question:
A bottle of pure n-heptane is accidentally poured into a drum of pure toluene in a laboratory. One of the laboratory assistants suggests that since heptane boils at a lower temperature than toluene, the following purification procedure can be used:
Pour the mixture (2 mol% n-heptane) into a simple still pot. Boil the mixture at 1 atm and condense the vapors until all heptane is boiled away. Obtain the pure toluene from the residue.
You, a chemical engineer with knowledge of vapor–liquid equilibrium, immediately realize that such a purification method will not work.
(a) Indicate this by a curve showing the composition of the material remaining in the still-pot after various quantities of the liquid have been distilled. What is the composition of the residue after 50 wt% of the original material has been distilled? What is the composition of the cumulative distillate?
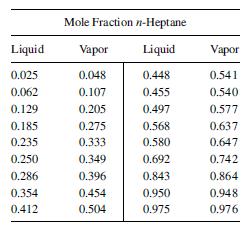
(b) When one-half of the heptane has been distilled, what is the composition of the cumulative distillate and the residue? What weight %t of the original material has been distilled? Equilibrium data at 1 atm [Ind. Eng. Chem., 42, 2912 (1949)] are:
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





