A vaporliquid mixture at 250F and 500 psia contains N 2 , H 2 S, CO 2
Question:
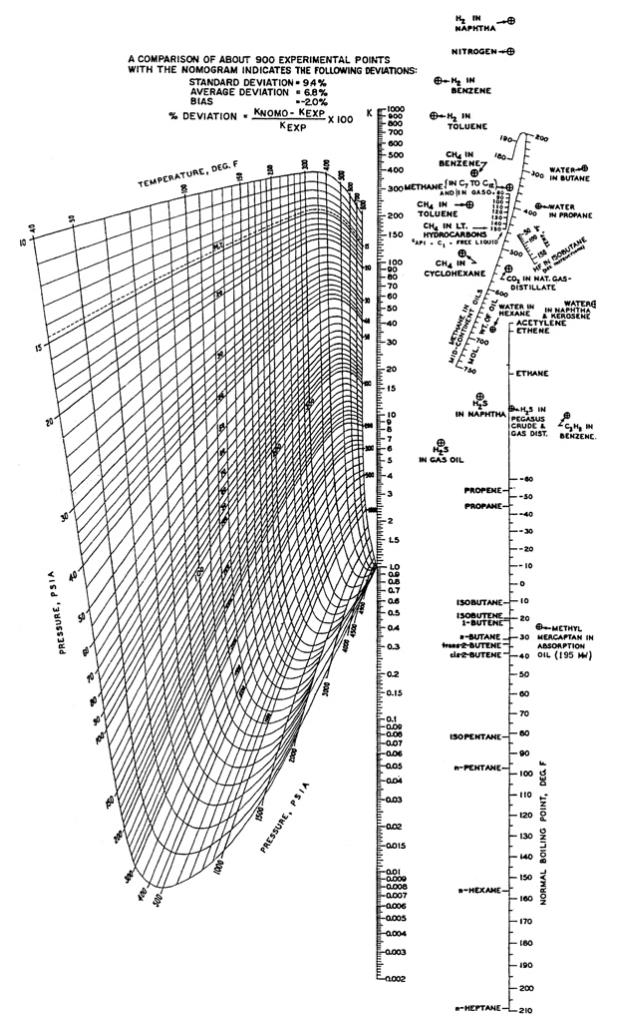
A vapor–liquid mixture at 250ºF and 500 psia contains N2, H2S, CO2, and all the normal paraffins from methane to heptane. Use Figure 2.4 to estimate the K-value of each component. Which components will be present to a greater extent in the equilibrium vapor?
Transcribed Image Text:
2 Cr PRESSURE, PSIA 3 A COMPARISON OF ABOUT 900 EXPERIMENTAL POINTS WITH THE NOMOGRAM INDICATES THE FOLLOWING DEVIATIONS 500. -005 STANDARDEVIATION 6.8% **20% BIAS % DEVIATION KNOMO-EXP X 100 Do PRESSURE, PSIA MID- NI ********** 'דידי 0 1 8 2 8 8 8 8 8 8 8 8 NORMAL BOILING POINT, DEG F 2 8 8 8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Answer The Kvalues of the components are given in the table be...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:
Students also viewed these Life Sciences questions
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
A water storage tank contains liquid and vapor in equilibrium at 110C. The distance from the bottom of the tank to the liquid level is 8 m. What is the absolute pressure at the bottom of the tank?
-
Five different laboratories participated in an interlaboratory study involving determinations of the iron level in water samples. The results below are replicate determinations of Fe in ppm for...
-
What are the strengths and weaknesses of e-distribution?
-
Survey accuracy According to the U.S. Census Bureau, Current Population Survey, Annual Social and Economic Supplement, the average income for females was \(\$ 28,466\), and the standard deviation was...
-
In a single stage impulse turbine the blade angles are equal and the nozzle angle is \(20^{\circ}\). The velocity coefficient for the blade is 0.83. Find the mzximum blade efficiency possible. If the...
-
Forced air at T = 25C and V = 10 m/s is used to cool electronic elements on a circuit board. One such element is a chip, 4 mm by 4 mm. located 120 mm from the leading edge of the board. Experiments...
-
A bank is loaning $5,000 for three years at an interest rate of 7.5 percent. How much additional interest can the bank earn if it compounds interest continuously rather than annually?
-
Capital Toys' management is considering eliminating product A, which has been showing a loss for several years. The company's annual income statement, in $000s, is as follows: a. Specific to each...
-
Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene (2) system at 45 C are L 12 = 0.124 and L 21 = 0.523. Use these with...
-
One thousand kmol/h of rich gas at 70F with 25% C 1 , 15% C 2 , 25% C 3 , 20% nC 4 , and 15% nC 5 by moles is to be absorbed by 500 kmol/h of nC 10 at 90F in an absorber at 4 atm. Calculate by the...
-
Find the Maclaurin series of f (by any method) and its radius of convergence. Graph f and its first few Taylor polynomials on the same screen. What do you notice about the relationship between these...
-
A harmonic wave traveling along a light string approaches a splice to a heavier string, as shown in Figure P16.16. Which changes as the wave crosses the boundary: wavelength, frequency, both, or...
-
Two sinusoidal waves travel in opposite directions along the same string. The wavelength and frequency are the same in both waves, and each has amplitude \(0.0289 \mathrm{~m}\). If there is a phase...
-
Two sinusoidal waves travel along the same string. Their time-dependent wave functions are \[\begin{aligned} & f_{1}(x, t)=a \sin \left(b x-q t-\frac{1}{4} \pi ight) \\ & f_{2}(x, t)=a \sin \left(b...
-
A \(100-\mathrm{m}\) steel cable that helps support the Golden Gate Bridge is \(72.0 \mathrm{~mm}\) in diameter and composed of 100 steel wires twisted together. Approximate this as a single uniform...
-
Use a bar graph to show the distribution of votes. To answer question, use the following results of the election to the General Assembly from one New Jersey district in 1991. Batten Lookabaugh...
-
Find the missing values. a. [13 23] + [-6 31] = [x y] b. c. d. e. 90 101090 101-[C11 C12 05 95 ]L05 95] C21 C22 18-231+-2.4 5.432.25.3 12.21-[a b 1011cd 18 231112 5.4 32.2 21 22 10 -2.4 12.21- 5.3...
-
The Ferris wheel in the figure has a radius of 68 feet. The clearance between the wheel and the ground is 14 feet. The rectangular coordinate system shown has its origin on the ground directly below...
-
How can RNAi gene silencing be used to determine the function of genes?
-
How do insertional mutagenesis approaches differ from other reverse genetic approaches?
-
Insertional mutagenesis is a powerful tool in both plants and animals. However, when performing large-scale insertional mutagenesis, what major advantage do plants have over animals?
-
A 3.0 resistor is connected across the terminals of a 100 V battery. If 0.50 A of current flows, what is the internal resistance of the battery?
-
The rate of blood flow through the aorta is Q = 100 cm/s. A capillary has an average cross sectional area of Acap = 3 x10 -11 m and supports a blood speed of cap = 1 mm/s. From this information, what...
-
A particle is trapped in a potential well described by U(x)=16-b where U is in joules, x is in meters, and b= 4.0 J. Find the force on the particle when it's at a) x=2.2m and b) x=-1.7m.

Study smarter with the SolutionInn App


