Question: For a mixture with mole fractions 0.005 methane, 0.595 ethane, and the balance n-butane at 50 psia, and using K-values from Figure 2.4: (a) Find
For a mixture with mole fractions 0.005 methane, 0.595 ethane, and the balance n-butane at 50 psia, and using K-values from Figure 2.4:
(a) Find the bubble-point temperature.
(b) Find the temperature that results in 25% vaporization at this pressure, and determine the liquid and vapor compositions in mole fractions.
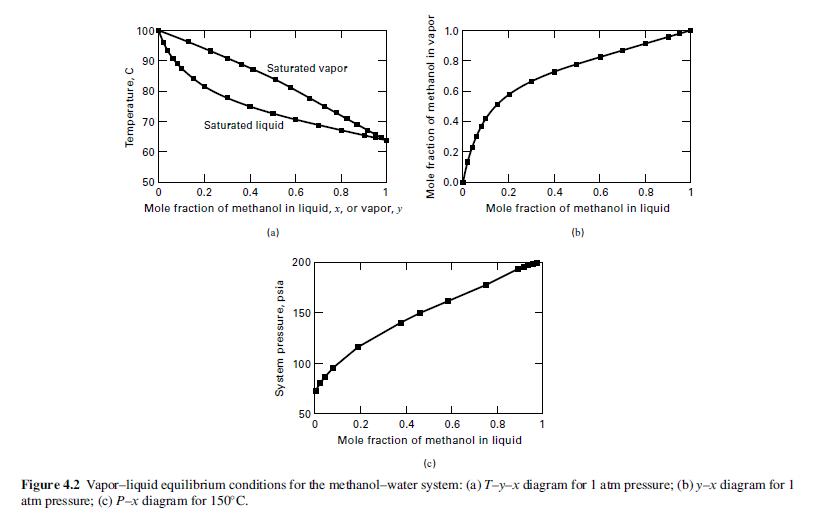
Temperature, C 100 90 80 70- 60 Saturated vapor 50 Saturated liquid 1 1 0 0.4 0.6 0.8 1 0.2 Mole fraction of methanol in liquid, x, or vapor, y (a) System pressure, psia 200 150 100 50 0 Mole fraction of methanol in vapor 1.0 0.8 0.6 0.4 0.2 0.0 0 1 1 0.8 0.2 0.4 0.6 Mole fraction of methanol in liquid (b) 1 1 0.8 0.2 0.4 0.6 Mole fraction of methanol in liquid 1 T 1 (c) Figure 4.2 Vapor-liquid equilibrium conditions for the methanol-water system: (a) T-y-x diagram for 1 atm pressure; (b) y-x diagram for 1 atm pressure; (c) P-x diagram for 150 C.
Step by Step Solution
3.32 Rating (173 Votes )
There are 3 Steps involved in it
a The bubblepoint temperature 10566F 7849K b ... View full answer

Get step-by-step solutions from verified subject matter experts


