Question: For the seven-phase equilibrium system shown in Figure 4.30, assume air consists of N 2 , O 2 , and argon. What is the number
For the seven-phase equilibrium system shown in Figure 4.30, assume air consists of N2, O2, and argon. What is the number of degrees of freedom? What variables might be specified?
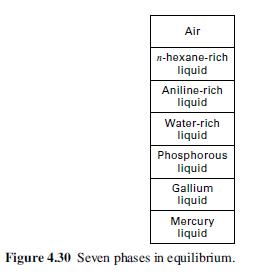
Air n-hexane-rich. liquid Aniline-rich liquid Water-rich liquid Phosphorous liquid Gallium liquid Mercury liquid Figure 4.30 Seven phases in equilibrium.
Step by Step Solution
★★★★★
3.55 Rating (162 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The number of degrees of freedom for a sevenphase equilibrium system is 6 The varia... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


