In Examples 15.13 and 15.18, benzene is adsorbed from air at 70 F in a 6-ft-high
Question:
In Examples 15.13 and 15.18, benzene is adsorbed from air at 70οF in a 6-ft-high bed of silica gel and then stripped with air at 145°F. If the bed height is changed to 30 ft, the following data are obtained for breakthrough at 641 minutes for the adsorption step:
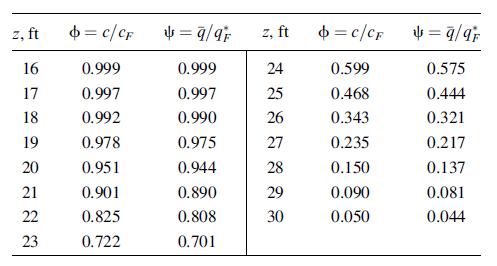
If the bed is regenerated isothermally with pure air at 1 atm and 145°F, and the desorption of benzene during the heat-up period is neglected, determine the loading, q, profile at a time sufficient to remove 90% of the benzene from the 30-ft bed if an interstitial pure air velocity of 98.5 ft/minute is used. Values of k and K at 145°F are given in Example 15.18.
Example 15.13
Air at 70°F and 1 atm, containing 0.9 mol% benzene, enters a fixedbed adsorption tower at 23.6 lb/minute. The tower is 2 ft in inside diameter and packed to a height of 6 ft with 735 lb of 4 x 6 mesh silica gel (SG) particles with a 0.26-cm effective diameter and an external void fraction of 0.5. The adsorption isotherm for benzene has been determined to be linear for the conditions of interest:
![]()
where q = lb benzene adsorbed per ft3 of silica gel particles, and c* = equilibrium concentration of benzene in the gas, in lb benzene per ft3 of gas.
Mass-transfer experiments simulating conditions in the 2-ft-diameter bed have been fitted to a linear-driving-force (LDF) model:
![]()
where time is in minutes and 0.206 is the constant k in minute-1, which includes resistances both in the gas film and in the adsorbent pores, with the latter resistance dominant.
Using the approximate concentration-profile equations of Klinkenberg [127], compute a set of breakthrough curves and the time when the benzene concentration in the exiting air rises to 5% of the inlet. Assume isothermal, isobaric operation. Compare breakthrough time with time predicted by the equilibrium model.
Example 15.18:
In Example 15.13, benzene is adsorbed from air at 70°F and 1 atm onto silica gel in a 6-ft-long fixed-bed adsorber. Breakthrough occurs at close to 97.1 minutes for ф = 0.05. At that time, values of ф = c/cF and Ψ = q̅/q*f in the bed are distributed as follows, where z' is measured backward from the bed exit for the adsorption step. These results were obtained by applying the numerical method just described, to the adsorption step, and are in close agreement with the Klinkenberg solution given in Example 15.13.
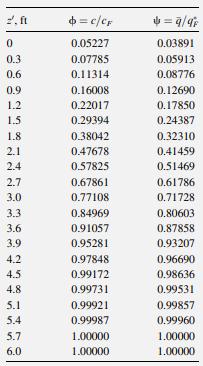
If the bed is regenerated isothermally with pure air at 1 atm and 145°F, and benzene desorption during the heat-up period is neglected, determine the loading, q, profile at times of 15, 30, and 60 minutes for air stripping at interstitial velocities of: (a) 197 ft/minute, and (b) 98.5 ft/minute. At 145°F and 1 atm, the adsorption isotherm, in the same units as in Example 15.13, is
![]()
giving an equilibrium loading of about 20% of that at 70°F. Assume that k is unchanged from the value of 0.206 in Example 15.13.
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





