Question: A 55 mol% propane and 45 mol% propylene gas mixture is to be separated into products containing 10 and 90 mol% propane by adsorption in
A 55 mol% propane and 45 mol% propylene gas mixture is to be separated into products containing 10 and 90 mol% propane by adsorption in a continuous, countercurrent system operating at 25°C and 1 atm. The adsorbent is silica gel, for which equilibrium data are given in Exercise 15.9. Determine by the McCabe–Thiele method the:
(a) adsorbent flow rate per 1,000 m3 of feed gas at 25°C and 1 atm for 1.2 times the minimum, and
(b) number of stages required.
Data From Exercise 15.9.
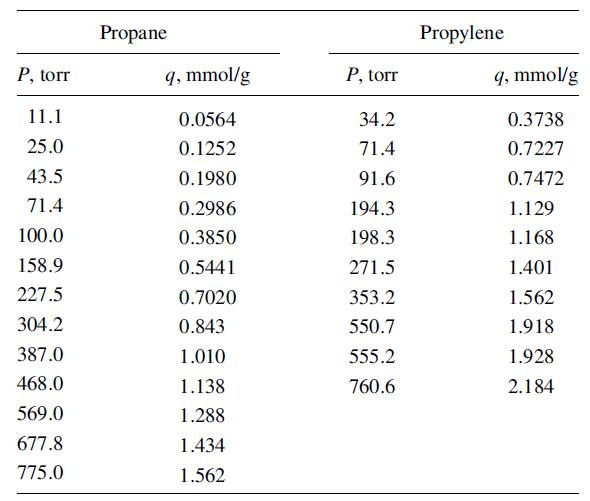
Lewis, Gilliland, Chertow, and Hoffman [J. Am. Chem. Soc., 72, 1153–1157 (1950)] measured adsorption equilibria for propane, propylene, and binary mixtures thereof on activated carbon and silica gel. Adsorbate capacity was high on carbon, but selectivity was poor. Selectivity was high on silica gel, but capacity was low. For silica gel (751 m2/g), the pure-component data below were obtained at 25°C:
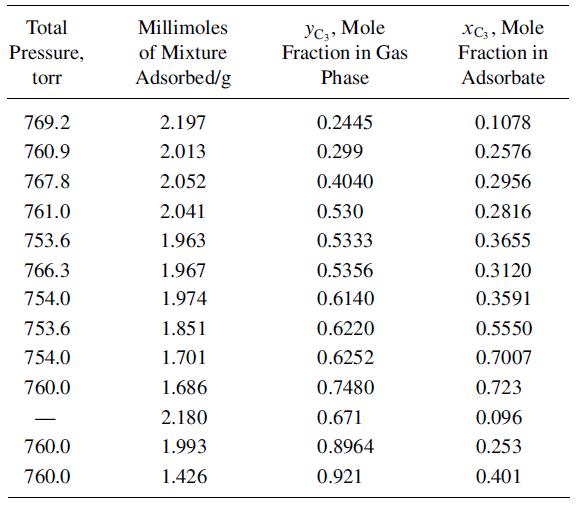
The following mixture data were measured with silica gel at 25°C, over a pressure range of 752–773 torr:
(a) Fit the pure-component data to Freundlich and Langmuir isotherms. Which gives the best fit? Which component is most strongly adsorbed?
(b) Use the results of the Langmuir fits in part
(a) to predict binary mixture adsorption using the extended-Langmuir equation, (15-32). Are the predictions adequate?
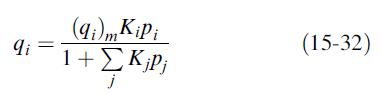
(c) Ignoring the pure-component data, fit the binary-mixture data to the extended- Langmuir equation, (15-32). Is the fit better than that obtained in part (b)?
(d) Ignoring pure-component data, fit the binary mixture data to the extended-Langmuir–Freundlich equation, (15-33). Is the fit adequate? Is it better than that in part (c)?
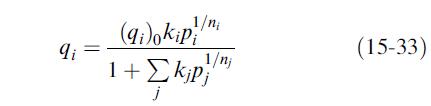
(e) For the binarymixture data, compute the relative selectivity, ![]() for each condition. Does a vary widely or is the assumption of constant a reasonable?
for each condition. Does a vary widely or is the assumption of constant a reasonable?
P, torr 11.1 25.0 43.5 71.4 100.0 158.9 227.5 304.2 387.0 468.0 569.0 677.8 775.0 Propane q, mmol/g 0.0564 0.1252 0.1980 0.2986 0.3850 0.5441 0.7020 0.843 1.010 1.138 1.288 1.434 1.562 P, torr 34.2 71.4 91.6 194.3 198.3 271.5 353.2 550.7 555.2 760.6 Propylene q, mmol/g 0.3738 0.7227 0.7472 1.129 1.168 1.401 1.562 1.918 1.928 2.184
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Answer a The Langmuir isotherm gives the best fit ... View full answer

Get step-by-step solutions from verified subject matter experts


