Liquid air is fed to the top of a perforated-tray reboiled stripper operated at 1 atm. Sixty
Question:
Liquid air is fed to the top of a perforated-tray reboiled stripper operated at 1 atm. Sixty % of the oxygen in the feed is to be drawn off in the bottoms vapor product, which is to contain 0.2 mol% nitrogen. Based on the assumptions and equilibrium data below, calculate:
(a) The mole % N2 in the vapor from the top plate,
(b) The vapor generated in the still per 100 moles of feed, and
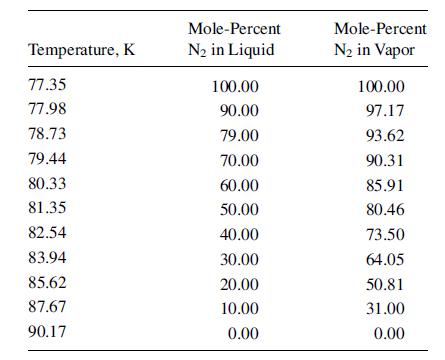
(c) The number of stages required. Assume constant molar overflow equal to the moles of feed. Liquid air contains 20.9 mol% O2 and 79.1 mol% N2. The equilibrium data [Chem. Met. Eng., 35, 622 (1928)] at 1 atm are:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:





