One hundred lbmol of 10 mol% propane, 30 mol% n-butane, 10 mol% n-pentane, and the balance n-hexane
Question:
One hundred lbmol of 10 mol% propane, 30 mol% n-butane, 10 mol% n-pentane, and the balance n-hexane is to be separated in a batch rectifier equipped with a still-pot, a total condenser with a liquid holdup of 1.0 ft3, and a column with the equivalent of eight theoretical stages and a total holdup of 0.80 ft3. The pressure in the condenser is 50.0 psia and the column pressure drop is 2.0 psi. The rectification campaign, given as follows, is designed to produce cuts of 98 mol% propane and 99.8 mol% n-butane, a residual cut of 99 mol% n-hexane, and two intermediate cuts, one of which may be a relatively rich cut of n-pentane. All five operating steps are conducted at a molar vapor boilup rate of 40 lbmol/h. Use a suitable batch-distillation computer program to determine the amounts and compositions of all cuts.
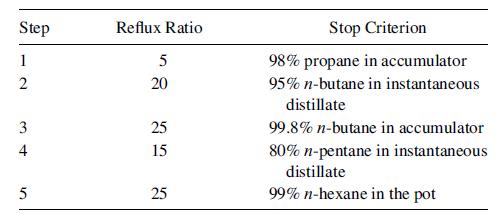
How might you alter the operation steps to obtain larger amounts of the product cuts and smaller amounts of the intermediate cuts?
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





