One hundred lbmol of benzene (B), monochlorobenzene (MCB), and o-dichlorobenzene (DCB) is distilled in a batch rectifier
Question:
One hundred lbmol of benzene (B), monochlorobenzene (MCB), and o-dichlorobenzene (DCB) is distilled in a batch rectifier that has a total condenser, a column with 10 theoretical stages, and a stillpot. Following establishment of total reflux, the first operation step begins at a boilup rate of 200 lbmol/h and a reflux ratio of 3. At the end of 0.60 h, the following conditions exist for the top three stages in the column:
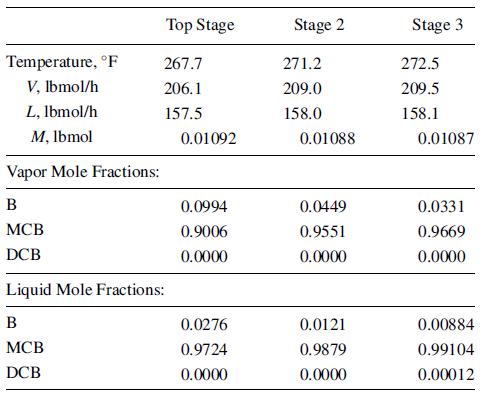
In addition, still-pot and condenser holdups at 0.6 h are 66.4 and 0.1113 lbmol, respectively. For benzene, use the preceding data with (13-36) and (13-39) to estimate the liquid-phase mole fraction of benzene leaving Stage 2 at 0.61 h by using the explicit-Euler method with a Dt of 0.01 h. If the result is unreasonable, explain why, with respect to stability and stiffness considerations.
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





