Question: Repeat the calculations of Example 12.1 but use 1 = methanol, 2 = water, and 3 = acetone. Are the results any different? If not,
Repeat the calculations of Example 12.1 but use 1 = methanol, 2 = water, and 3 = acetone. Are the results any different? If not, why not? Prove your conclusion mathematically.
EXAMPLE 12.1
This example is similar to Example 11.5.1 on page 283 of Taylor and Krishna [15]. The following results were obtained for Tray n from a rate-based calculation of a ternary distillation at 14.7 psia, involving acetone
(1), Methanol
(2), And water
(3) In a 5.5-ft-diameter column using sieve trays with a 2-inch-high weir. Vapor and liquid phases are assumed to be completely mixed.
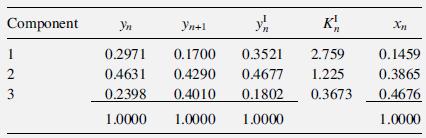
The computed products of the gas-phase, binary mass-transfer coefficients and interfacial area, using the Chan–Fair correlation of §6.6, are as follows in lbmol/(h-unit mole fraction):
k12 = k21 = 1; 955; k13 = k31 = 2; 407; k23 = k32 = 2; 797
(a) Compute the molar diffusion rates.
(b) Compute the masstransfer rates.
(c) Calculate the Murphree vapor-tray efficiencies.
Component 1 23 Yn y K 0.2971 0.1700 0.3521 2.759 0.4631 0.4290 0.4677 1.225 0.2398 0.4010 0.1802 0.3673 1.0000 1.0000 1.0000 Yn+1 Xn 0.1459 0.3865 0.4676 1.0000
Step by Step Solution
3.57 Rating (161 Votes )
There are 3 Steps involved in it
Solution a The molar diffusion rates are calculated from the equations d12 k12y1 y2 d13 k13y1 y3 d23 ... View full answer

Get step-by-step solutions from verified subject matter experts


