The following results were obtained at tray n from a rate-based calculation at 14.7 psia, for a
Question:
The following results were obtained at tray n from a rate-based calculation at 14.7 psia, for a ternary mixture of acetone
(1), Methanol
(2), And water
(3) In a sieve-tray column assuming that both phases are perfectly mixed.
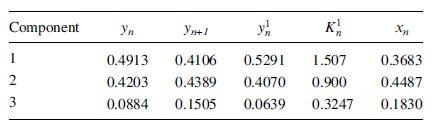
The products of the computed gas-phase, binary mass-transfer coefficients, and interfacial area from the Chan–Fair correlations are as follows in units of lbmol/(h-unit mole fractions).
k12 = k21 = 1;750; k13 = k31 = 2;154; k23 = k32 = 2;503
The vapor rates are Vn = 1,200 lbmol/h and Vn+1 = 1,164 lbmol/h.
Determine:
(a) Component molar diffusion rates;
(b) Mass-transfer rates;
(c) Murphree vapor-tray efficiencies.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:





