Using the enthalpy-concentration diagram of Figure 7.37, determine the following for a mixture of n-hexane (H) and
Question:
Using the enthalpy-concentration diagram of Figure 7.37, determine the following for a mixture of n-hexane (H) and n-octane (O) at 1 atm:
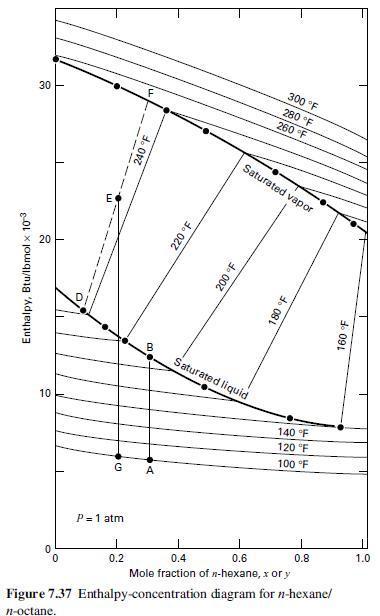
(a) Temperature and compositions of equilibrium liquid and vapor resulting from adiabatic mixing of 950 lb/h of a mixture of 30 mol% H in O at 180°F with 1,125 lb/h of a mixture of 80 mol% H in O at 240οF;
(b) Energy required to partially condense, by cooling, a mixture of 60 mol% H in O from an initial temperature of 260°F to 200°F. What are the compositions and amounts of the resulting vapor and liquid phases per lbmol of original mixture?
(c) If the vapor from part (b) is further cooled to 180°F, determine the compositions and relative amounts of the resulting vapor and liquid.
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





