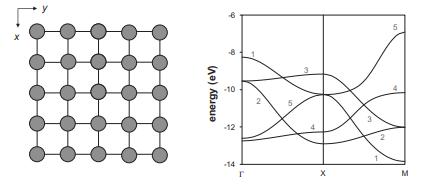
A 2D square lattice of 3d transition-metal atoms 2.3 apart has the band structure shown below
Question:
A 2D square lattice of 3d transition-metal atoms 2.3 Å apart has the band structure shown below (the contributions of the 4s and 4p orbitals have been omitted to simplify the analysis).
(a) Considering orbital overlap for each of the five 3d orbitals at Γ, X, and M, characterize the nearest-neighbor interactions for each band as (σ, π, or δ) bonding, antibonding, or nonbonding at each of these k points.
(b) Use your answers from part (a) to associate bands numbered 1–3 in the diagram above with a dxy, dxz, or dyz orbital (due to similar behavior at Γ, X, andM, bands 4–5 have contributions from both dx2−y2 and dz2).
(c) Which d-electron count will provide the strongest metal–metal bonding: d1, d4, or d8?
Step by Step Answer:

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt





