Question: Calculate the self-diffusion coefficient of Fe in Problem 3.22, given the unit-cell parameter a = 2.87 . Problem 3.22 Assume that bcc iron at 1800
Calculate the self-diffusion coefficient of Fe in Problem 3.22, given the unit-cell parameter a = 2.87 Å.
Problem 3.22
Assume that bcc iron at 1800 K has a fraction of vacant Fe sites of 0.0001, an Fe atom vibration frequency
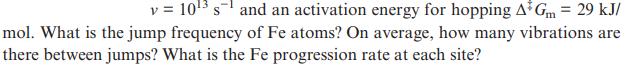
v = 10 s and an activation energy for hopping A Gm = 29 kJ/ mol. What is the jump frequency of Fe atoms? On average, how many vibrations are there between jumps? What is the Fe progression rate at each site?
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Equation 318 suggests multiplying the prog... View full answer

Get step-by-step solutions from verified subject matter experts


