Consider the band structure for an infinite 1D chain of equally spaced titanium and oxygen atoms shown
Question:
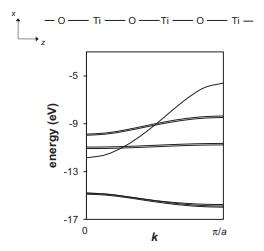
Consider the band structure for an infinite 1D chain of equally spaced titanium and oxygen atoms shown below, with an overall stoichiometry of TiO. In this calculation, only the Ti 3d orbitals and the O 2p orbitals have been taken into account.
(a) Identify the orbital making the largest contribution to the band that spans the energy range from approximately −12 eV to −6 eV.
(b) Identify the orbitals that make the largest contribution to the two flat bands located at roughly −11 eV.
(c) Indicate the approximate location of the Fermi level.
(d) Would you expect this chain to be a metal or a semiconductor?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





