Question: Construct an MO diagram for a linear H 2 O molecule by analogy with BeH 2 in Figure 5.17. (a) Determine the degeneracy and orbital
Construct an MO diagram for a linear H2O molecule by analogy with BeH2 in Figure 5.17.
(a) Determine the degeneracy and orbital character of the HOMO and the LUMO.
(b) Identify the orbitals on oxygen that participate in bonding to hydrogen.
(c) Now distort the molecule by bending the H–O–H bond and consider how this impacts the MO diagram. How does the orbital character of the HOMO(s) change?
(d) Which oxygen orbitals now participate in bonding?
(e) Is this distortion an example of a firstor second-order Jahn–Teller distortion?
Figure 5.17
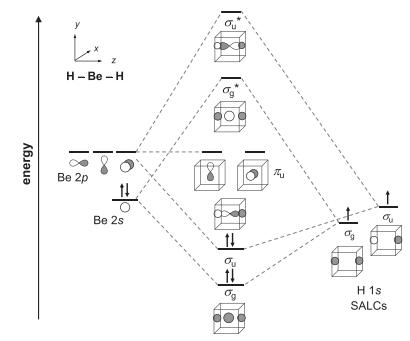
energy L H-Be-H Be 2p Z Be 2s 14 ou og The H 1s SALCS.
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
a The HOMO is the doubly degenerate set of nonbonding O 2p orbitals The ... View full answer

Get step-by-step solutions from verified subject matter experts


