Question: NiO adopts the cubic NaCl-type structure while PtO adopts the cooperite structure shown below. (a) What factor do you think is responsible for the differing
NiO adopts the cubic NaCl-type structure while PtO adopts the cooperite structure shown below.
(a) What factor do you think is responsible for the differing crystal chemistry preferences of these two compounds? Consider the splitting and occupation of the d orbitals for each compound.
(b) Which structure type do you think PdO will adopt? Would it be possible to tell from a magnetic measurement?
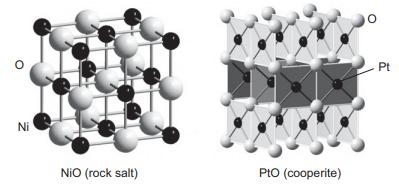
Ni NiO (rock salt) PtO (cooperite) O Pt
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
Figure 525 Figure 72 a Pt is larger than Ni so it is not possible to attribute the lower coordinatio... View full answer

Get step-by-step solutions from verified subject matter experts


