Prove that Equation (15.6) reduces to the LorenzLorentz equation (15.4) in the case of purely ionic bonding.
Question:
Prove that Equation (15.6) reduces to the Lorenz–Lorentz equation (15.4) in the case of purely ionic bonding.
Equation (15.6)
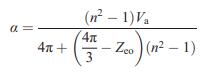
Equation (15.4)
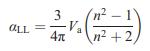
Transcribed Image Text:
a = 4x + (n - 1) Va 4 3 -Zeo (1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Purely ionic bonding implie...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
To calculate variance and standard deviation, we take the deviations from the mean. At times, we need to consider the deviations from a target value rather than the mean. Consider the case of a...
-
A registered dealer, based in Chandigarh, makes a supply to another registered dealer located in Chandigarh, valuing rupees 1,20,000. The applicable rate of GST is 12%. Calculate the amount of tax...
-
In aqueous solution, hydrogen sulfide reduces (a) Fe3+ to Fe2+ (b) Br2 to Br- (c) MNO4- to Mn2+ (d) HNO3 to NO2. In all cases, under appropriate conditions, the product is elemental sulfur. Write a...
-
Suppose that the probability that a mechanic fixes a car correctly is 0.9. Determine the odds against the mechanic fixing a car correctly.
-
Calculate the Lande g factor for atoms (a) In S states; (b) In singlet states.
-
Find the center of gravity (xÌ, yÌ) of a mass of density f(x, y) = 1 in the given region R. h R
-
Show that after nearly all of the positrons were annihilated and the electron number density had nearly leveled off at the proton density, the ratio of the positron number density to the photon...
-
The following transactions relate to the City of Monticello for the fiscal year ended June 30, 2013. Prepare (a) All the journal entries necessary to record these transactions, and identify the fund(...
-
Suppose that Jane believes that lying is wrong, whereas Joe believes that lying is not wrong. What does Error imply about this disagreement?
-
Use the AndersonEggleton relationship to calculate the refractive index of the mineral orthoclase (KAlSi 3 O 8 ) with a unit-cell volume 720.4 3 containing four formula units. The cation...
-
Yellow light of the Na doublet with a wavelength of 589.30.3 nm and frequency of 5.0910 14 Hz in vacuum enters Fe 2 O 3 that has a refractive index of 3.00. Calculate the speed, wavelength, and...
-
Suppose that two continuous random variables X and Y have a joint probability density function f(x, y) = A(x-3)y for -2 < x < 3 and 4 < y < 6, and f(x, y) = 0 elsewhere. (a) What is the value of A?...
-
UPS, a delivery services company, has a Beta of 1.10, and Wal-Mart has a Beta of 0.70. The Risk-Free Rate of return is 4.50% and the expected return of the market portfolio is 11.50%. What is the...
-
A4 A solar heater is installed on the roof of a house. At noon, it can heat up water from 20 C to 50 C when water flows through it at a rate of 2 kg/min. The specific heat capacity of water is 4200 J...
-
Think about a Emma Watson whom you consider your role model of a leader. 1) What makes you admire this Emma Waston: their mindset? values? personality traits? methods? achievements? 2) Talk to any...
-
Using a Counter-Controlled While Loop To prompt the user to create 3 usernames and passwords. (JAVA)?
-
Submit a1 java program that use SENTINEL Create a secure java grocery store application program that prompts the user to enter as many gorcery prices ant items as they would like and when they are...
-
Robert Helmer and Percy Helmer, Jr., were authorized signatories on the corporate checking account of Event Marketing, Inc. The Helmers signed a check drawn on Event Marketings account and issued to...
-
Question 2 For an n x n matrix A = form) via (aij)
-
An alkyne with molecular formula C 5 H 8 was treated with sodium in liquid ammonia to give a disubstituted alkene with molecular formula C 5 H 10 . Draw the structure of the alkene.
-
Predict the major product expected for each of the following reactions: (a) (b) (c) (d) (e) (f) ? XS HCI CI 1) xs NANH/NH3 2) H,0 CI
-
Suggest reagents that would achieve the following transformation: CI CI CI
-
How would you plan to learn more about the health care system and the role of nurses in another country? What are critical elements in the sustainability of global health efforts? You are considering...
-
What are the potential challenges or barriers that organizations may face when implementing human resource planning, and how can they overcome them?
-
Why are quantitative skills so valuable in business disciplines? How important do you think quantitative skills will be in your career? Explain.

Study smarter with the SolutionInn App


