Question: Use the ClausiusMossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO 2 (rutile-type, P4 2 /mnm, Z = 2, a =
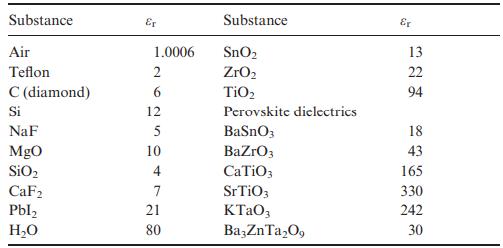
Use the Clausius–Mossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO2 (rutile-type, P42/mnm, Z = 2, a = 4.74 Å, c = 3.19 Å), TiO2 (rutile-type, a = 4.59 Å, c = 2.96 Å) and ZrO2 (baddeleyite-type, P21/c, Z = 4, unit-cell volume = 141 Å). Does the Clausius–Mossotti equation give a reasonably accurate estimate for each compound when compared to the experimental values of εr given in Table 8.1? If not, what is the origin of the discrepancy?
Equation (8.13)
![a= V 4 2 Er (+2) [CGSes : a in , V, in A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/2/1/12165ae4a61b74551705921119175.jpg)
Table 8.2
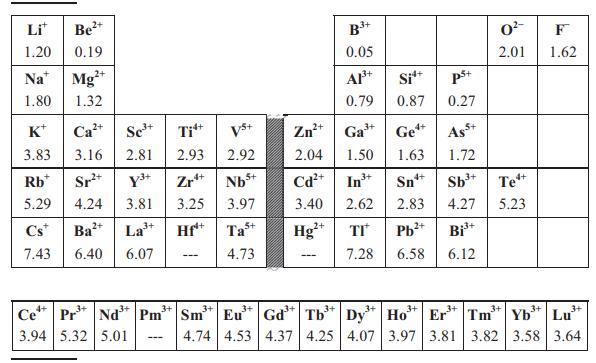
Table 8.1
a= V 4 2 Er (+2) [CGSes : a in , V, in A]
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Calculated values are r 13 SnO 2 r 20 ZrO 2 and r 43 TiO 2 Th... View full answer

Get step-by-step solutions from verified subject matter experts


