A displacement reaction is an oxidation-reduction reaction in which one element displaces another from solution. In each
Question:
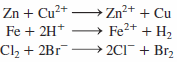
Transcribed Image Text:
Zn + Cu2+ Fe + 2H* Cl, + 2Br- Zn²+ + Cu Fe2+ + H2 2CI+ Br, 111
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (10 reviews)
Oxidized ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A disproportionation reaction is an oxidation-reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
-
Complete and balance the following half-reactions. In each case indicate whether the half-reaction is an oxidation or a reduction.
-
Complete and balance the following half-reactions. In each case indicate whether the half-reaction is an oxidation or a reduction. Discuss.
-
You have been asked by the principal investigator of a qualitative research project to suggest ideas for maintaining confidentiality of private, sensitive information obtained from human...
-
Halle describes "Third-worldism" as an automatic resistance to change among poorer countries to proposals that come from richer countries. What could be done to overcome this distrust?
-
(a) Evaluate (2, 1) and (2.1, 1.05) and calculate z (b) Use the total differential dz to approximate z. (x, y) = 4x + 2y
-
A viscous fluid is poured onto a horizontal plate as shown in Fig. P7.27. Assume that the time, \(t\), required for the fluid to flow a certain distance, \(d\), along the plate is a function of the...
-
Wyandotte Company provided the following information for the last calendar year: Beginning inventory: Direct materials ..... $25,900 Work in process ..... 44,700 Ending inventory : Direct materials...
-
You have just completed the appraisal of an office building and have concluded that the market value of the property is $3,500,000. You expect Potential Gross Income (PGI) in the first year of...
-
In which of the four types of organizational cultures- family, Eiffel Tower, guided missile, or incubator- would most people in the United States feel comfortable? In which would most Japanese feel...
-
When magnesium is placed in an acid solution, hydrogen gas is given off. Is magnesium or hydrogen the better reducing agent?
-
Hydrogen sulfide gas dissolves in water and dissociates very slightly: H 2 S 2H + + S 2 -. How would the acidity of the solution be affected by a. Increasing the pressure of H 2 S? b. Raising the...
-
The Co(NH3)63+ ion is diamagnetic, but Fe(H2O)62+ is paramagnetic. Explain.
-
From the following ledger balances, prepare a trial balance for the Cheyenne Corp. at June 3 0 , 2 0 2 2 . All account balances are normal.Accounts Payable $ 1 0 , 0 0 0 , Cash $ 7 , 4 0 0 , Common...
-
Compounds A to F are intermediates in a catalytic cycle. iPr2 ipr2 ipr2 P CI Ph Pd Pd H CI PiPr2 Pr2 C pd-p Prz A ipr2 I Pd iprz Prz CI 9.5 Ph D B ipr2 Pd PrP- ipr2 CI -P Pd. Ph Ph Prz Ph F E
-
The idea that employment rents are an incentive for employees to work harder is illustrated in a study by Edward Lazear (an economic advisor to former US President George W. Bush) and his co-authors....
-
Desperate for a job out of public health school, you have taken a position with a health foods company. It has produced a new product called Super Holistic Chakra Enhancer, which it claims reduces...
-
-1.9(1) g (t) ait, where g is a differentiable function. The table above gives selected values for f, g, and g'. If h is the function given by h (a)=2-e+3, for which Let f be the function given by...
-
You are asked to use your best judgment to estimate the probability that there will be a nuclear war within the next 10 years. Is this an example of relative frequency or subjective definition of...
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
A vessel containing a liquid is opened inside an evacuated chamber. Will you see a liquidgas interface if the volume of the initially evacuated chamber is a. Less than the critical volume, b. A...
-
Calculate S R for the reaction H 2 (g) + Cl 2 (g) 2HCl (g) at 870. K. Omit terms in the temperature dependent heat capacities higher than T 2 /K 2 .
-
Use the result of Problem P3.10 to derive a formula for (CV /V ) T for a gas that obeys the RedlichKwong equation of state, RT 1 a Vm - b VT VVm + b)' T VVm P:
-
The following Python program swaps two variables which is entered by user # To take input from the user x = input ('Enter value of x: ') y = input ('Enter value of y: ') # create a temporary variable...
-
1. Raman purchases a motor car from Bharathan whose cash price is Rs. 56,000 on 11.93. Rs. 15,000 is paid on signing the contract and the balance is to be paid in three equal annual instalments of...
-
Write a function that takes in a value x, a value el, and a list and adds as many el's to the end of the list as there are x's in the list. Make sure to modify the original list using list mutation...

Study smarter with the SolutionInn App


