Consider the following mixture of SO 2 (g) and O 2 (g). If SO 2 (g) and
Question:
Consider the following mixture of SO2(g) and O2(g).
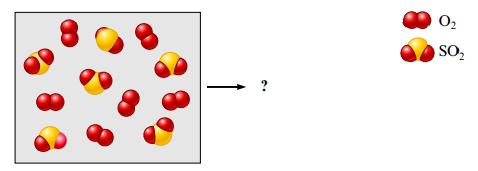
If SO2(g) and O2(g) react to form SO3(g), draw a representation of the product mixture assuming the reaction goes to completion. What is the limiting reactant in the reaction? If 96.0 g of SO2 react with 32.0 g O2, what mass of product will form?
Transcribed Image Text:
? 0₂ SO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a The limiting reactan...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What is a limiting reactant in a reaction mixture? Explain how it determines the amount of product.
-
What is a limiting reactant problem? Explain the method you are going to use to solve limiting reactant problems.
-
Describe how you could separate the following mixture of metal ions: Cd2+, Pb2+, and Sr2+.
-
The number x of bicycle helmets people are willing to buy per week from a retail chain at a price of $p is given by x = 1,000 - 60p + 25 20 ¤ p ¤ 100 (see the figure). (A) Find dx/dp....
-
Because expenses incurred both in a business and for the production of investment income are deductible, why is it important to determine in which category a particular activity falls?
-
Create a portfolio of at least two options on the same futures and evaluate the position in terms of the position's OPM value, delta, gamma, and theta. On the OVME screen, click the + icon next to...
-
What is the most accepted input power required for an ICP accelerometer?
-
New York Beverages, Inc., has three plants that make and bottle cola, lemon-lime, and miscellaneous flavored beverages, respectively. The raw materials, labor costs, and automated technology are...
-
Four full adders are employed to form a 4-bit circuit shown in the below figure. Interpret the function of control line C and the outputs R3-Ro & R4. Assume that A & B are unsigned integers and A>B....
-
The Lexington Group has the following unadjusted trial balance as of May 31, 2018: The debit and credit totals are not equal as a result of the following errors: A. The cash entered on the trial...
-
You know that chemical A reacts with chemical B. You react 10.0 g A with 10.0 g B. What information do you need to determine the amount of product that will be produced? Explain.
-
Consider an iron bar on a balance as shown. As the iron bar rusts, which of the following is true? Explain your answer. a. The balance will read less than 75.0 g. b. The balance will read 75.0 g. c....
-
1. Did the decedent have testamentary capacity? 2. Did the decedent act of his own free will in cutting out his son and the sons family, or was Ingas influence too great? 3. How should the court rule...
-
In purchasing, when someone acts with authority that they don't formally possess, is this known as apparent or actual authority?
-
In purchasing, a bid or cost estimate provided by a potential supplier is commonly called a what?
-
Who has greater authority, a sales representative or a sales agent?
-
What is the holistic approach, and how is it related to anthropology and its four subfields, as well as to other disciplines?
-
Interpret the statement, Every human is like all other humans, some other humans, and no other human.
-
A homologous DNA region, which was 20,000 bp in length, was sequenced among four different species. The following number of nucleotide differences were obtained: Construct a phylogenetic tree that...
-
Write electron configurations for the following ions, and determine which have noble-gas configurations: (a) Cd2+ (b) p3- (c) Zr4+ (d) Ru3+ (e) As3- (f) Ag+
-
Prove that the perfect gas temperature scale and the thermodynamic temperature scale based on the Second Law of thermodynamics differ from each other by at most a constant numerical factor.
-
Evaluate (ClS/ClV)]' for (a) A van der Waals gas, (b) A Dieterici gas (Table 1.7). For an isothermal expansion, for which kind of gas (and a perfect gas) will /).5be greatest? Explain your conclusion.
-
Two of the four Maxwell relations were derived in the text, but two were not. Complete their derivation by showing that (S/V)T = (p/T)V (T/P)s = (V/S)p
-
Explain why leasing is an option for a company expansion. include, what leasing is and how it will benefit the company in it's expanding efforts. Also, how is capital or operating leasing recorded on...
-
Discuss the following statement: " A head of state signs a treaty on behalf of his country in excess of authority of his country, such treaty shal be void for inconsistency with domestic law of the...
-
A company is looking at new equipment with an installed cost of $415,329. This cost will be depreciated straight-line to zero over the project's 5-year life, at the end of which the equipment can be...

Study smarter with the SolutionInn App


