Ethyl acetate is synthesized in a nonreacting solvent (not water) according to the following reaction: For the
Question:
Ethyl acetate is synthesized in a nonreacting solvent (not water) according to the following reaction:
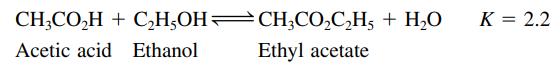
For the following mixtures (a–d), will the concentration of H2O increase, decrease, or remain the same as equilibrium is established?
a. [CH3CO2C2H5] = 0.22 M, [H2O] = 0.10 M, [CH3CO2H] = 0.010 M, [C2H5OH] = 0.010 M
b. [CH3CO2C2H5] = 0.22 M, [H2O] = 0.0020 M, [CH3CO2H] = 0.0020 M, [C2H5OH] = 0.10 M
c. [CH3CO2C2H5] = 0.88 M, [H2O] = 0.12 M, [CH3CO2H] = 0.044 M, [C2H5OH] = 6.0 M
d. [CH3CO2C2H5] = 4.4 M, [H2O] = 4.4 M, [CH3CO2H] = 0.88 M, [C2H5OH] = 10.0 M
e. What must the concentration of water be for a mixture with [CH3CO2C2H5] = 2.0 M, [CH3CO2H] = 0.10 M, and [C2H5OH] = 5.0 M to be at equilibrium?
f. Why is water included in the equilibrium expression for this reaction?
Step by Step Answer:

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste





