Question: We expect the atomic radius to increase going down a group in the periodic table. Can you suggest why the atomic radius of hafnium breaks
We expect the atomic radius to increase going down a group in the periodic table. Can you suggest why the atomic radius of hafnium breaks this rule? (See data below.)
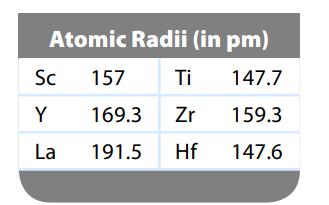
Atomic Radii (in pm) 147.7 159.3 147.6 Sc Y La 157 169.3 191.5 Ti Zr Hf
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


