A rigid tank contains 5 kg of O 2 , 8 kg of N 2 , and
Question:
A rigid tank contains 5 kg of O2, 8 kg of N2, and 10 kg of CO2 at 100 kPa and 1000 K. Assume that the mixture behaves as an ideal gas. Determine
(a) The mass fraction of each component,
(b) The mole fraction of each component,
(c) The partial pressure of each component,
(d) The average molar mass (apparent molecular weight) and the gas constant of the mixture,
(e) The volume of the mixture in m3.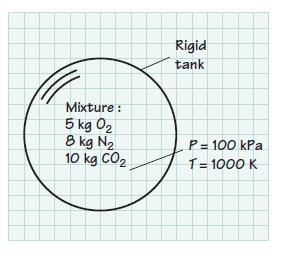
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





