Consider the equilibrium dissociation of carbon dioxide CO 2 CO + 1/2 O 2 . At
Question:
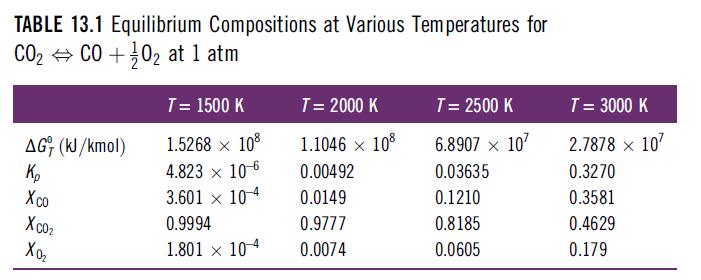
Consider the equilibrium dissociation of carbon dioxide CO2 ⇔ CO + 1/2 O2. At 2500 K, the equilibrium constant is 0.03635. Calculate the enthalpy of reaction for this reaction at 2500 K and use this to estimate the equilibrium constant for a temperature of 3000 K. Compare your estimated value with the exact value from Table 13.1 in Example 13.2 and discuss.
Table 13.1 in Example 13.2
Transcribed Image Text:
TABLE 13.1 Equilibrium Compositions at Various Temperatures for CO₂ CO +02 at 1 atm AGT (kJ/kmol) Кр Xco Xcoz Xo₂ T = 1500 K 1.5268 x 108 4.823 x 10-6 3.601 104 0.9994 1.801 x 104 T = 2000 K 1.1046 × 108 0.00492 0.0149 0.9777 0.0074 T = 2500 K 6.8907 x 107 0.03635 0.1210 0.8185 0.0605 T = 3000 K 2.7878 x 10² 0.3270 0.3581 0.4629 0.179
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
To calculate the enthalpy of reaction for the equilibrium dissociation of carbon dioxide at 2500 K w...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The data in the table concern the lactonization of hydroxyvaleric acid at 25oC. They give the concentration C(t)of this acid in moles per liter after minutes. (a) Find the average rate of reaction...
-
Consider the reaction 2 CO2 2 CO + O2 obtained after heating 1 kmol CO2 to 3000 K. Find the equilibrium constant from the shift in Gibbs function and verify its value with the entry in Table A.11....
-
This question draws from the tables in the previous question. Let us try to get an idea of what it would cost an American family at todays prices to purchase the bundle consumed by an average Swedish...
-
Rice and Flower were partners sharing profit and loss equally. Statement of Financial Position as at 31 December 2020. Non current Assets Premises Machinery Vehicles Fittings Current Assets Inventory...
-
Form groups of three to six students each. Each student should pick two companies, preferably from different industries. Find the appropriate data and compute the market-to-book ratio and the ROE for...
-
what is the long - run market ( or aggregate ) supply for the whole industry? The individual firm s long - run market supply is determined at p MC = = 1 2 ?
-
A critical width dimension on an integrated circuit board was measured on 100 boards. The ordered measurements are: Given the specification limits \(L S L=2.496\) and \(U S L=2.516\), evaluate the...
-
Winter Corporation has just completed its comparative statements for the year ended December 31, 2012. At this point, certain analytical and interpretive procedures are to be undertaken. The...
-
Losing to a Weaker Foe What began as a heavily conventional military campaign to unseat the regime of Saddam Hussein had become a bitter, unconventional struggle against frustrated Sunnis who...
-
1. Compare the two financing options in terms of projected return on the owners equity investment. Ignore any effect from income taxes. 2. What if Dalton is wrong and the company earns only 4 percent...
-
Ammonia is used as a refrigerant in large-scale refrigeration systems. Using data for ammonia (NH 3 ) from the NIST resources, verify that the condition for phase equilibrium (Eqs.13.35) is met. Use...
-
1. Consolidation workpaper entries normally: a Are posted to the general ledger accounts of one or more of the affiliates b Are posted to the general ledger accounts only when the financial statement...
-
Why should enterprisers focus on goals and values as they prepare to start their new ventures?
-
Five errors have been found in the records of A V Kingfisher and require correction by general journal. a Courier charges paid through the petty cash book $55 ($50 + $5 GST) were posted in the...
-
In the accounts of A Bottlebrush, a tax invoice for the sale of stock $385 ($350 +$35 GST) to Glen Bawn was processed through the sales journal incorrectly as $836 ($760 + $76 GST). Control accounts...
-
Two machines were purchased in the financial year to 30 June 2022. Machine 1 is depreciated using the diminishing balance method at 35% p.a. It was purchased and installed on 31 January 2022 for $44...
-
Compute the taxable income for 2019 under each of the following circumstances: a. Jim is married and files a joint return. Jim and his wife have two dependent children. They have adjusted gross...
-
During the last few years, many companies have suffered major trading losses because of the poor economic climate. Assume your firm has found itself in this situation and is considering a major...
-
Identify the five accounts receivable balance- related audit objectives. For each objective, list one audit procedure.
-
How does the organizational structure of an MNC influence its strategy implementation?
-
An engineer is studying the mileage performance characteristics of five types of gasoline additives. In the road test he wishes to use cars as blocks; however, because of a time constraint, he must...
-
Construct a set of orthogonal contrasts for the data in Problem 4-27. Compute the sum of squares for each contrast. Problem 4-27. An engineer is studying the mileage performance characteristics of...
-
Seven different hardwood concentrations are being studied to determine their effect on the strength of the paper produced. However, the pilot plant can only produce three runs each day. As days may...
-
You will use information from this entry in your presentation. Include the following for Netflix: List the benefits of data administration compared to database administration for Netflix. Propose an...
-
Search online for tutorial topics related to database administration or SQL for beginners. Summarize the two best sources. Explain why you think these tutorials are the best.
-
Kaspersky Security Center 10 was installed with the Standard installation settings. The administrator wants to add the capability to manage Kaspersky Security for Windows Server. What is the...

Study smarter with the SolutionInn App


